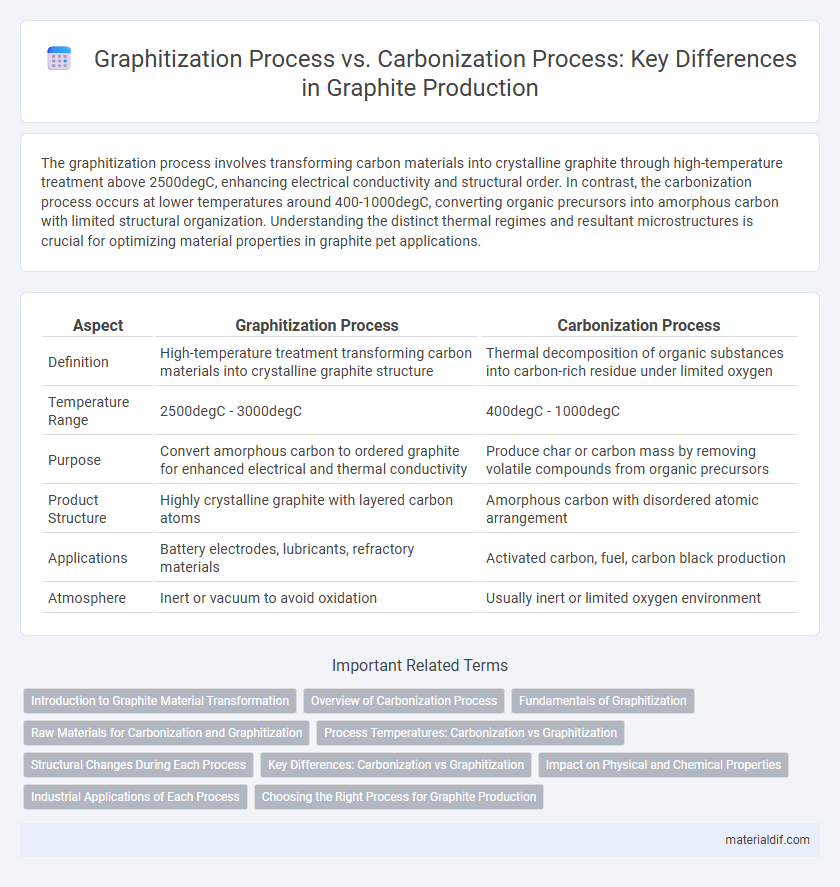
The graphitization process involves transforming carbon materials into crystalline graphite through high-temperature treatment above 2500degC, enhancing electrical conductivity and structural order. In contrast, the carbonization process occurs at lower temperatures around 400-1000degC, converting organic precursors into amorphous carbon with limited structural organization. Understanding the distinct thermal regimes and resultant microstructures is crucial for optimizing material properties in graphite pet applications.
Table of Comparison
| Aspect | Graphitization Process | Carbonization Process |
|---|---|---|
| Definition | High-temperature treatment transforming carbon materials into crystalline graphite structure | Thermal decomposition of organic substances into carbon-rich residue under limited oxygen |
| Temperature Range | 2500degC - 3000degC | 400degC - 1000degC |
| Purpose | Convert amorphous carbon to ordered graphite for enhanced electrical and thermal conductivity | Produce char or carbon mass by removing volatile compounds from organic precursors |
| Product Structure | Highly crystalline graphite with layered carbon atoms | Amorphous carbon with disordered atomic arrangement |
| Applications | Battery electrodes, lubricants, refractory materials | Activated carbon, fuel, carbon black production |
| Atmosphere | Inert or vacuum to avoid oxidation | Usually inert or limited oxygen environment |
Introduction to Graphite Material Transformation
Graphitization involves the structural transformation of amorphous carbon into crystalline graphite through high-temperature heat treatment, enhancing electrical conductivity and thermal stability. Carbonization, by contrast, converts organic precursors into carbon-rich solids by decomposing non-carbon elements at lower temperatures, yielding a primarily amorphous carbon structure. The graphitization process refines the carbon's microstructure, critical for advanced materials in aerospace, energy storage, and electronic applications.
Overview of Carbonization Process
The carbonization process involves the thermal decomposition of organic material at temperatures typically between 400degC and 1000degC in an inert atmosphere, leading to the formation of a carbon-rich solid by driving off volatile compounds. This process is crucial for producing carbon fibers, charcoal, and coke, serving as a precursor stage before graphitization. Carbonization enhances the material's carbon content and structural stability, setting the foundation for subsequent high-temperature graphitization to develop crystalline graphite structures.
Fundamentals of Graphitization
Graphitization is a high-temperature process that transforms amorphous carbon materials into crystalline graphite by reorganizing carbon atoms into a hexagonal lattice structure, enhancing electrical conductivity and thermal stability. Unlike the carbonization process, which primarily removes volatile components from organic precursors to form a carbonaceous residue, graphitization involves extensive atomic rearrangement and graphitic domain growth that occurs above 2500degC. The fundamental mechanism of graphitization depends on the progressive alignment of graphene layers facilitated by temperature-induced diffusion and the elimination of structural defects.
Raw Materials for Carbonization and Graphitization
Raw materials for carbonization typically include coal, petroleum coke, and biomass, which undergo thermal decomposition to produce carbon-rich char. In contrast, graphitization requires the use of high-purity carbon precursors like petroleum coke or needle coke that can structurally rearrange into crystalline graphite at temperatures above 2500degC. The carbonization process removes volatile components and increases carbon content, while graphitization transforms the amorphous carbon obtained into highly ordered graphite layers.
Process Temperatures: Carbonization vs Graphitization
Carbonization process occurs at temperatures between 400degC and 1000degC, where organic materials decompose to form char with a largely amorphous carbon structure. Graphitization process takes place at much higher temperatures, typically above 2500degC, transforming amorphous carbon into crystalline graphite with well-ordered graphene layers. The significant difference in process temperatures determines the degree of structural ordering and the resulting physical properties of the carbon material.
Structural Changes During Each Process
The graphitization process involves the transformation of carbonaceous materials into crystalline graphite through high-temperature treatment above 2500degC, resulting in the reorganization of disordered carbon atoms into a well-ordered hexagonal lattice. In contrast, the carbonization process occurs at lower temperatures (up to 1200degC), where organic materials decompose into amorphous carbon with limited structural order and the release of volatile compounds. Structural changes during graphitization increase the degree of graphitic crystallinity and reduce defects, whereas carbonization primarily removes non-carbon elements without significant graphitic rearrangement.
Key Differences: Carbonization vs Graphitization
Carbonization is the thermal decomposition of organic material at temperatures between 400degC and 1,000degC, resulting in a carbon-rich residue with amorphous structure and volatile byproducts. Graphitization occurs at higher temperatures above 2,500degC, transforming amorphous carbon into crystalline graphite with layered hexagonal lattice structure, significantly enhancing electrical conductivity and thermal stability. The key difference lies in the structural transformation where carbonization produces non-graphitic carbon, while graphitization generates highly ordered graphite crystals.
Impact on Physical and Chemical Properties
Graphitization and carbonization processes differ significantly in their impact on physical and chemical properties of carbon materials. Graphitization enhances crystallinity, resulting in a highly ordered graphite structure with increased electrical conductivity and thermal stability, while carbonization produces amorphous carbon with lower density and less structural order. The chemical reactivity and mechanical strength are markedly improved through graphitization due to the development of graphitic layers, unlike the more reactive and porous nature of carbonized materials.
Industrial Applications of Each Process
Graphitization involves the transformation of carbon materials into high-purity graphite through high-temperature treatment above 2500degC, making it essential for producing electrodes, lubricants, and refractory materials in industries such as metallurgy and electronics. Carbonization, conducted at lower temperatures between 400degC and 1000degC, converts organic precursors into carbon-rich char used predominantly in activated carbon production, fuel manufacturing, and steelmaking processes. Industrial applications leverage graphitization for enhanced electrical conductivity and thermal stability, while carbonization is favored for cost-effective carbonaceous material synthesis and energy production.
Choosing the Right Process for Graphite Production
Selecting the appropriate process between graphitization and carbonization hinges on the desired purity and structural properties of the graphite. Graphitization involves high-temperature treatment above 2500degC to convert amorphous carbon into crystalline graphite, yielding superior electrical conductivity and thermal stability essential for advanced industrial applications. Carbonization, with temperatures typically below 1200degC, produces a carbon-rich material with less ordered structure, suitable for applications where high purity and crystallinity are not critical.
Graphitization Process vs Carbonization Process Infographic
 materialdif.com
materialdif.com