Flake graphite features a layered, crystalline structure that offers higher purity and excellent electrical conductivity, making it ideal for industrial applications such as batteries and lubricants. In contrast, amorphous graphite lacks a well-defined crystal structure, resulting in lower purity and reduced conductivity but is often more abundant and cost-effective for uses like brake linings and pencils. Choosing between flake and amorphous graphite depends on performance requirements and budget constraints in specific industries.
Table of Comparison
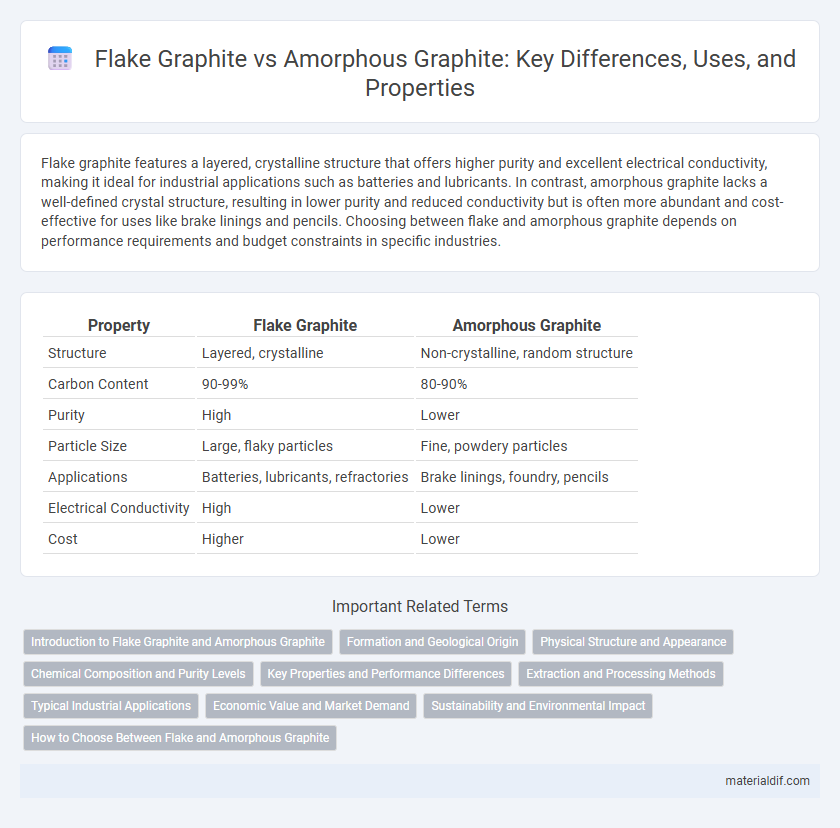
| Property | Flake Graphite | Amorphous Graphite |
|---|---|---|
| Structure | Layered, crystalline | Non-crystalline, random structure |
| Carbon Content | 90-99% | 80-90% |
| Purity | High | Lower |
| Particle Size | Large, flaky particles | Fine, powdery particles |
| Applications | Batteries, lubricants, refractories | Brake linings, foundry, pencils |
| Electrical Conductivity | High | Lower |
| Cost | Higher | Lower |
Introduction to Flake Graphite and Amorphous Graphite
Flake graphite consists of flat, plate-like particles with high crystallinity, making it ideal for applications requiring excellent conductivity and lubricity, such as batteries and refractories. Amorphous graphite, characterized by its fine, less organized structure, offers cost-effective use in products like brake linings and foundry facings due to its abundance and lower purity. Understanding the distinction between flake and amorphous graphite is crucial for selecting the appropriate type based on performance needs and industrial applications.
Formation and Geological Origin
Flake graphite forms through metamorphic processes where carbon-rich sediments undergo high temperature and pressure, resulting in crystalline, layered structures often found in schist and gneiss formations. Amorphous graphite originates from highly altered coal deposits or organic-rich sediments subjected to low-grade metamorphism, leading to a fine-grained, non-crystalline structure typically associated with sedimentary basins. Geological settings for flake graphite are mainly regional metamorphic belts, whereas amorphous graphite is prevalent in sedimentary basins with abundant organic material.
Physical Structure and Appearance
Flake graphite exhibits a crystalline structure composed of hexagonal carbon layers arranged in a stacked, plate-like formation, giving it a shiny, metallic luster with visible flake particles. In contrast, amorphous graphite lacks a well-defined crystalline arrangement, appearing as fine, powdery particles with a dull, matte finish due to its irregular and disordered carbon structure. The distinct physical structures of flake and amorphous graphite directly influence their appearance, with flake graphite displaying larger, reflective flakes while amorphous graphite appears more granular and non-reflective.
Chemical Composition and Purity Levels
Flake graphite contains a higher carbon content, typically above 90%, resulting in superior purity levels compared to amorphous graphite, which generally has carbon content ranging from 80% to 85%. The crystalline structure of flake graphite contributes to its higher purity and uniform chemical composition, whereas amorphous graphite's fine particles exhibit a more heterogeneous composition with greater impurities. These differences in chemical composition and purity significantly impact the performance and suitability of graphite types for industrial applications such as batteries and lubricants.
Key Properties and Performance Differences
Flake graphite features a crystalline structure with high purity, excellent electrical and thermal conductivity, and superior lubricity, making it ideal for applications like batteries and lubricants. Amorphous graphite, characterized by a less ordered structure, typically has lower purity and conductivity but offers good lubrication and is cost-effective for powder metallurgy and brake linings. The key performance difference lies in flake graphite's enhanced electrical conductivity and higher grade purity versus the more abundant, lower-grade but versatile amorphous graphite.
Extraction and Processing Methods
Flake graphite is primarily extracted through open-pit or underground mining, followed by flotation to concentrate the larger, crystalline flakes, resulting in high-purity graphite suitable for battery and refractory applications. Amorphous graphite is often sourced from lower-grade coal or natural deposits and undergoes crushing, grinding, and flotation to obtain a fine powder with lower crystallinity and fewer technological uses. Processing flake graphite requires careful separation to preserve flake size, while amorphous graphite processing emphasizes particle size reduction and impurity removal to enhance its energy storage and lubrication properties.
Typical Industrial Applications
Flake graphite is commonly used in refractory materials, lubricants, and battery anodes due to its high purity and crystalline structure, which provides excellent thermal and electrical conductivity. Amorphous graphite, characterized by its less ordered carbon structure, is typically utilized in brake linings, pencils, and foundry facings for its good lubricity and lower production costs. Industrial applications favor flake graphite for high-performance needs while amorphous graphite serves cost-effective uses where extreme purity is not critical.
Economic Value and Market Demand
Flake graphite commands a higher economic value due to its superior purity, larger particle size, and extensive applications in batteries, lubricants, and refractory materials, driving strong market demand globally. Amorphous graphite, with lower carbon content and smaller particle size, is less costly but primarily used in lower-grade industrial products such as pencils and brake linings. Market trends show increasing investment in flake graphite mining and processing to meet the growing needs of electric vehicle batteries and energy storage systems.
Sustainability and Environmental Impact
Flake graphite, known for its higher purity and larger particle size, typically requires less energy-intensive processing compared to amorphous graphite, reducing its overall carbon footprint and environmental impact. Its natural crystalline structure allows for more efficient recycling and minimal chemical usage, supporting sustainable production practices. Conversely, amorphous graphite's lower purity demands extensive purification, resulting in higher energy consumption and increased emissions, challenging its environmental sustainability.
How to Choose Between Flake and Amorphous Graphite
Choosing between flake graphite and amorphous graphite depends primarily on the application's required purity, particle size, and thermal conductivity. Flake graphite offers higher purity and larger particle size, making it ideal for applications like refractories and batteries, while amorphous graphite, with its finer particles and lower cost, is better suited for lubricants and brake linings. Evaluating factors such as application type, desired physical properties, and budget constraints ensures optimal performance and cost-effectiveness in selecting the appropriate graphite form.
Flake Graphite vs Amorphous Graphite Infographic
 materialdif.com
materialdif.com