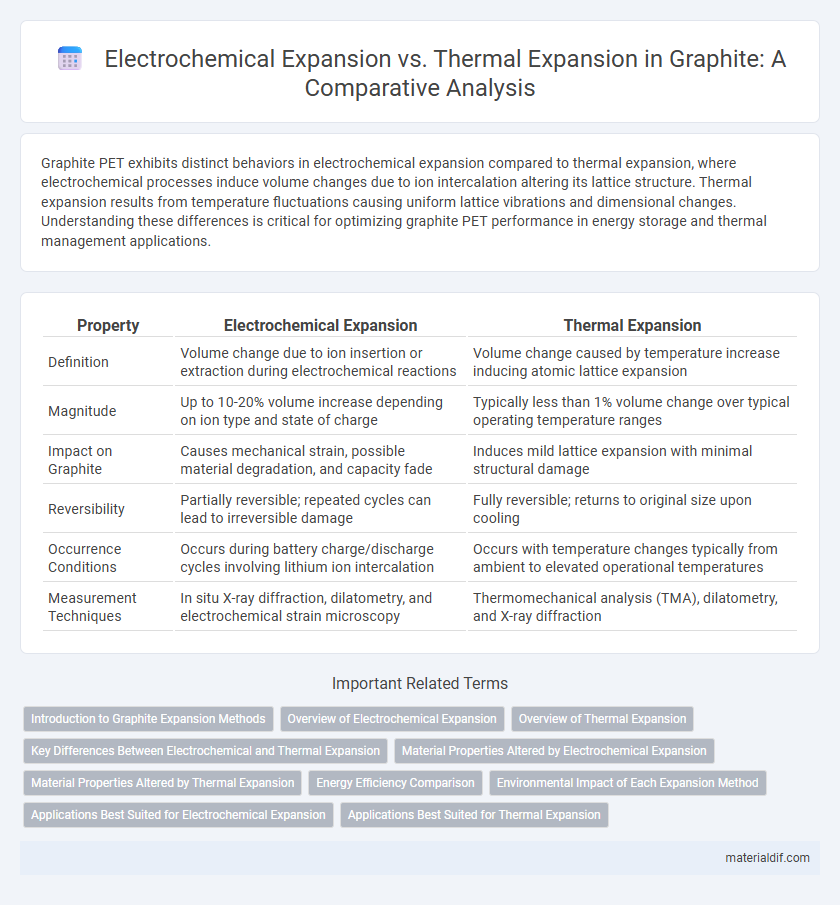
Graphite PET exhibits distinct behaviors in electrochemical expansion compared to thermal expansion, where electrochemical processes induce volume changes due to ion intercalation altering its lattice structure. Thermal expansion results from temperature fluctuations causing uniform lattice vibrations and dimensional changes. Understanding these differences is critical for optimizing graphite PET performance in energy storage and thermal management applications.
Table of Comparison
| Property | Electrochemical Expansion | Thermal Expansion |
|---|---|---|
| Definition | Volume change due to ion insertion or extraction during electrochemical reactions | Volume change caused by temperature increase inducing atomic lattice expansion |
| Magnitude | Up to 10-20% volume increase depending on ion type and state of charge | Typically less than 1% volume change over typical operating temperature ranges |
| Impact on Graphite | Causes mechanical strain, possible material degradation, and capacity fade | Induces mild lattice expansion with minimal structural damage |
| Reversibility | Partially reversible; repeated cycles can lead to irreversible damage | Fully reversible; returns to original size upon cooling |
| Occurrence Conditions | Occurs during battery charge/discharge cycles involving lithium ion intercalation | Occurs with temperature changes typically from ambient to elevated operational temperatures |
| Measurement Techniques | In situ X-ray diffraction, dilatometry, and electrochemical strain microscopy | Thermomechanical analysis (TMA), dilatometry, and X-ray diffraction |
Introduction to Graphite Expansion Methods
Graphite expansion methods primarily involve electrochemical expansion and thermal expansion, each exploiting different mechanisms to increase interlayer spacing. Electrochemical expansion utilizes ion intercalation, where ions insert between graphite layers causing volumetric swelling, often applied in battery and supercapacitor technologies. Thermal expansion relies on rapid heating to vaporize intercalated compounds, generating gases that force layers apart and produce expanded graphite with enhanced surface area and conductivity.
Overview of Electrochemical Expansion
Electrochemical expansion in graphite occurs when lithium ions intercalate between graphene layers during battery cycling, causing volumetric changes that affect anode stability. This expansion is governed by the reversible insertion and extraction of lithium ions, leading to strain and potential mechanical degradation over repeated charge-discharge cycles. Understanding electrochemical expansion is critical for improving the durability and performance of lithium-ion batteries using graphite anodes.
Overview of Thermal Expansion
Thermal expansion in graphite refers to the dimensional changes that occur when the material is subjected to temperature variations, primarily driven by the vibration of carbon atoms within its hexagonal lattice. This expansion is anisotropic, with in-plane expansion coefficients significantly lower than those along the c-axis due to the strong covalent bonds within graphene layers and weaker van der Waals forces between them. Understanding the thermal expansion behavior is essential for applications where graphite components experience high temperatures, as it influences structural integrity, thermal stress distribution, and overall material performance.
Key Differences Between Electrochemical and Thermal Expansion
Electrochemical expansion in graphite occurs due to ion intercalation during battery cycling, causing lattice distortion and volume increase primarily along the c-axis. Thermal expansion in graphite results from temperature-induced lattice vibrations, leading to anisotropic expansion with a greater effect along the a-axis compared to the c-axis. Key differences include the driving forces--electrochemical reactions versus temperature changes--and the anisotropy and magnitude of lattice parameter adjustments unique to each process.
Material Properties Altered by Electrochemical Expansion
Electrochemical expansion in graphite significantly alters its material properties by inducing lattice distortion and increased porosity, which reduces electrical conductivity and mechanical strength. Unlike thermal expansion, which causes uniform dimensional changes primarily due to temperature fluctuations, electrochemical expansion results from ion intercalation processes that disrupt the graphite's layered structure. These alterations can lead to capacity fading and structural degradation in electrochemical applications such as lithium-ion batteries.
Material Properties Altered by Thermal Expansion
Thermal expansion in graphite causes anisotropic dimensional changes due to its layered crystal structure, primarily expanding more along the c-axis than the a-axis. This expansion alters key material properties such as electrical conductivity and mechanical strength, often reducing the former due to increased interlayer spacing and inducing internal stresses that can weaken structural integrity. In contrast, electrochemical expansion involves volume changes from ion intercalation, which can cause more significant and less reversible modifications compared to the relatively predictable and reversible nature of thermal expansion.
Energy Efficiency Comparison
Electrochemical expansion in graphite typically exhibits higher energy efficiency compared to thermal expansion due to lower energy input requirements during ion intercalation and deintercalation processes. Thermal expansion involves increased lattice vibrations at elevated temperatures, resulting in greater energy loss through heat dissipation. In energy storage applications, minimizing thermal expansion enhances cycle stability and reduces energy consumption, making electrochemical methods more efficient for maintaining structural integrity in graphite electrodes.
Environmental Impact of Each Expansion Method
Electrochemical expansion of graphite primarily involves ion intercalation causing volume changes, which can lead to electrolyte degradation and waste generation, raising concerns about chemical disposal and environmental safety. Thermal expansion, driven by temperature fluctuations, results in physical stress without introducing additional chemicals, offering a relatively lower environmental footprint. Understanding the environmental impact of each method is crucial for sustainable graphite applications in energy storage and industrial processes.
Applications Best Suited for Electrochemical Expansion
Electrochemical expansion in graphite is predominantly utilized in energy storage devices like lithium-ion batteries, where precise control of volume changes during ion intercalation enhances cycle life and capacity retention. This phenomenon is critical in designing electrodes that undergo reversible expansion without mechanical degradation. Applications in supercapacitors and sensors also benefit from electrochemical expansion due to its ability to facilitate rapid, controllable volume modulation at the nanoscale.
Applications Best Suited for Thermal Expansion
Thermal expansion in graphite is ideal for applications requiring dimensional stability under high-temperature conditions, such as in nuclear reactors, heat exchangers, and high-temperature furnace components. Graphite's low thermal expansion coefficient combined with excellent thermal conductivity makes it suitable for environments where precise thermal management is critical. Electrochemical expansion, while useful in batteries and sensors, is less advantageous in such thermal-intensive industrial applications.
Electrochemical Expansion vs Thermal Expansion Infographic
 materialdif.com
materialdif.com