Graphite pets exhibit exceptional electrochemical performance due to their high electrical conductivity and stable intercalation properties, making them ideal for various energy storage applications. Mechanical strength is enhanced by the pet's layered structure, ensuring durability and resistance to deformation under stress. Balancing electrochemical efficiency and mechanical robustness is critical for optimizing the lifespan and reliability of graphite-based devices.
Table of Comparison
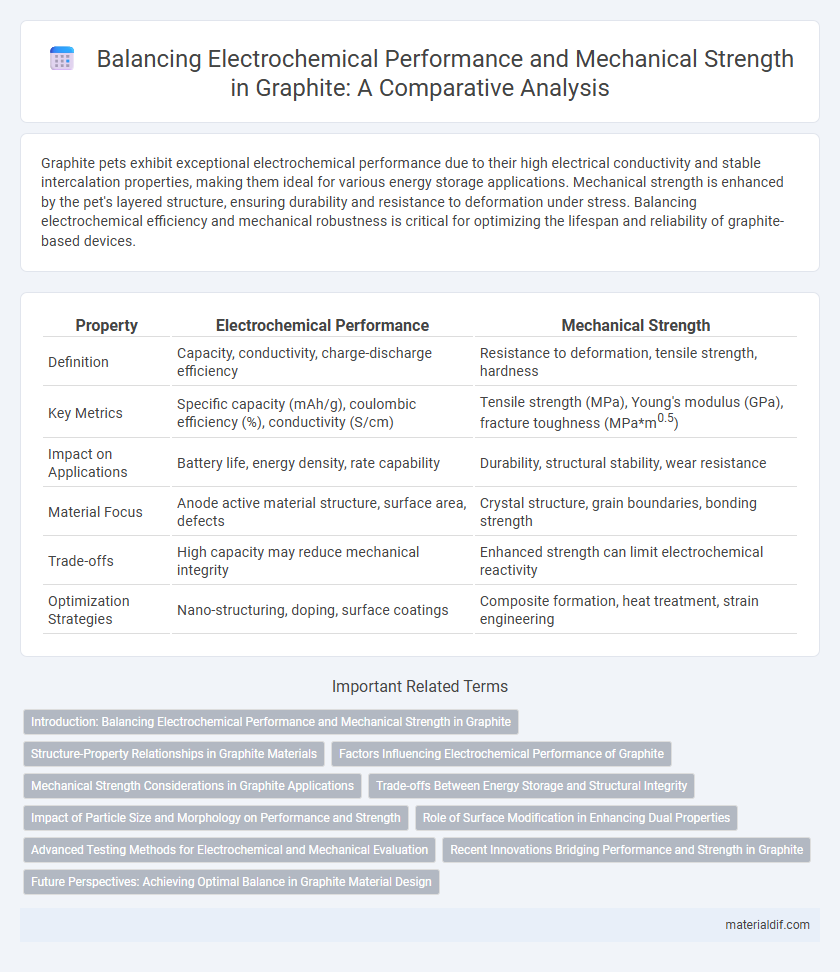
| Property | Electrochemical Performance | Mechanical Strength |
|---|---|---|
| Definition | Capacity, conductivity, charge-discharge efficiency | Resistance to deformation, tensile strength, hardness |
| Key Metrics | Specific capacity (mAh/g), coulombic efficiency (%), conductivity (S/cm) | Tensile strength (MPa), Young's modulus (GPa), fracture toughness (MPa*m0.5) |
| Impact on Applications | Battery life, energy density, rate capability | Durability, structural stability, wear resistance |
| Material Focus | Anode active material structure, surface area, defects | Crystal structure, grain boundaries, bonding strength |
| Trade-offs | High capacity may reduce mechanical integrity | Enhanced strength can limit electrochemical reactivity |
| Optimization Strategies | Nano-structuring, doping, surface coatings | Composite formation, heat treatment, strain engineering |
Introduction: Balancing Electrochemical Performance and Mechanical Strength in Graphite
Graphite's electrochemical performance critically depends on its structural integrity, where high mechanical strength ensures durability during charge-discharge cycles. Optimizing the balance between mechanical strength and electrochemical activity enhances battery lifespan and efficiency, particularly in lithium-ion applications. Key parameters such as crystallinity, particle size, and binder interaction directly influence both the mechanical robustness and the capacity retention of graphite electrodes.
Structure-Property Relationships in Graphite Materials
Graphite's electrochemical performance and mechanical strength are intrinsically linked to its layered crystal structure, which facilitates high electrical conductivity and ion intercalation while maintaining structural integrity. The anisotropic nature of graphite allows for efficient electron transport parallel to the basal planes, enhancing battery capacity and rate capability, whereas defects and grain boundaries influence mechanical durability and fracture resistance. Optimizing the microstructure, including layer stacking order and defect density, is critical for balancing electrochemical activity with mechanical robustness in applications such as lithium-ion batteries and flexible electronics.
Factors Influencing Electrochemical Performance of Graphite
Electrochemical performance of graphite is primarily influenced by factors such as particle size, crystallinity, and surface area, which affect lithium-ion intercalation and diffusion rates. Structural defects and the degree of graphitization determine electrical conductivity and cyclic stability, directly impacting battery efficiency and lifespan. Moreover, electrolyte compatibility and electrode porosity play crucial roles in optimizing ionic transport and minimizing capacity fade during charge-discharge cycles.
Mechanical Strength Considerations in Graphite Applications
Mechanical strength in graphite directly influences its durability and reliability in electrochemical applications such as batteries and fuel cells. High mechanical stability ensures dimensional integrity under repeated stress cycles, preventing electrode degradation and maintaining consistent electrochemical performance. Optimizing graphite's microstructure, including particle size and porosity, enhances its mechanical properties without compromising conductivity or capacity.
Trade-offs Between Energy Storage and Structural Integrity
Graphite electrodes in energy storage devices exhibit a critical trade-off between electrochemical performance and mechanical strength, where high energy capacity often compromises structural integrity. Optimizing graphite's microstructure enhances lithium-ion diffusion rates but may reduce fracture toughness, affecting durability under cycling stress. Balancing these factors is essential for developing long-lasting batteries with reliable capacity retention and resistance to mechanical degradation.
Impact of Particle Size and Morphology on Performance and Strength
Graphite's electrochemical performance and mechanical strength are significantly influenced by particle size and morphology, where smaller particles enhance electrode surface area, improving charge capacity and rate capability. Morphological features such as flake-like or spherical shapes affect mechanical integrity by dictating stress distribution and structural cohesion within composite electrodes. Optimizing these parameters enables a balance between high conductivity and robust mechanical durability essential for long-lasting energy storage devices.
Role of Surface Modification in Enhancing Dual Properties
Surface modification of graphite materials significantly enhances both electrochemical performance and mechanical strength by improving electrode-electrolyte interaction and reinforcing structural integrity. Techniques such as chemical functionalization, coating with conductive polymers, and plasma treatment introduce active sites that boost charge capacity while simultaneously increasing resistance to mechanical stress and fracture. Optimizing surface properties ensures balanced dual advantages essential for advanced battery and supercapacitor applications.
Advanced Testing Methods for Electrochemical and Mechanical Evaluation
Advanced testing methods for graphite include electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) to evaluate electrochemical performance, providing insights into charge transfer resistance and capacity retention. Mechanical strength is assessed using nanoindentation and tensile testing, which measure hardness, elasticity, and fracture toughness at micro and macro scales. Combining these techniques allows for a comprehensive understanding of the interplay between graphite's electrochemical stability and structural integrity in battery applications.
Recent Innovations Bridging Performance and Strength in Graphite
Recent innovations in graphite materials have significantly enhanced electrochemical performance while maintaining superior mechanical strength through advanced nano-engineering techniques and composite formulations. The integration of graphene oxide and carbon nanotubes has optimized ion transport pathways and structural integrity, leading to improved cycle stability and higher capacity retention in batteries. These breakthroughs enable graphite electrodes to deliver both high-rate performance and durability, addressing previous trade-offs between electrochemical efficiency and mechanical resilience.
Future Perspectives: Achieving Optimal Balance in Graphite Material Design
Future advancements in graphite material design aim to achieve an optimal balance between electrochemical performance and mechanical strength by engineering hierarchical structures and surface modifications. Nanostructured graphite composites incorporating conductive polymers and carbon nanotubes demonstrate enhanced lithium-ion diffusion rates alongside improved fracture toughness. Tailoring defect density and interlayer spacing at the atomic scale holds promise for next-generation graphite anodes with superior cycling stability and mechanical resilience.
Electrochemical Performance vs Mechanical Strength Infographic
 materialdif.com
materialdif.com