High purity graphite offers superior electrical conductivity and chemical resistance compared to standard purity graphite, making it ideal for demanding industrial applications. The reduced impurities in high purity graphite ensure enhanced performance in batteries, semiconductors, and aerospace components. Standard purity graphite, while more cost-effective, is typically suited for general uses where extreme precision and durability are not critical.
Table of Comparison
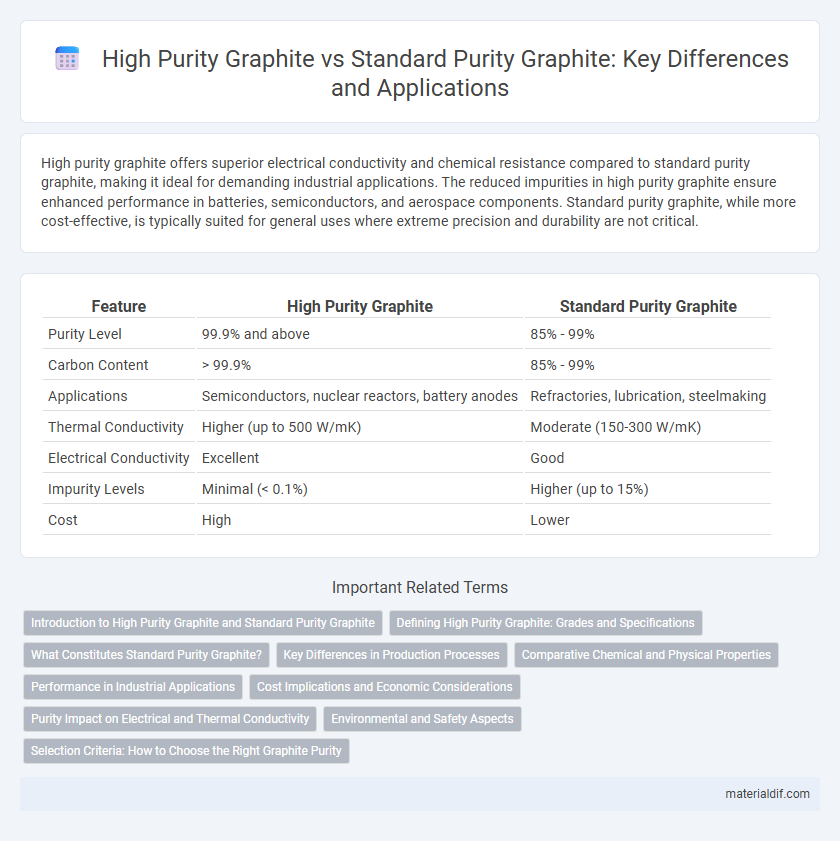
| Feature | High Purity Graphite | Standard Purity Graphite |
|---|---|---|
| Purity Level | 99.9% and above | 85% - 99% |
| Carbon Content | > 99.9% | 85% - 99% |
| Applications | Semiconductors, nuclear reactors, battery anodes | Refractories, lubrication, steelmaking |
| Thermal Conductivity | Higher (up to 500 W/mK) | Moderate (150-300 W/mK) |
| Electrical Conductivity | Excellent | Good |
| Impurity Levels | Minimal (< 0.1%) | Higher (up to 15%) |
| Cost | High | Lower |
Introduction to High Purity Graphite and Standard Purity Graphite
High purity graphite exhibits a carbon content exceeding 99.95%, distinguished by minimal impurities that enhance its electrical conductivity and thermal resistance, making it crucial for advanced technological applications such as semiconductors and nuclear reactors. Standard purity graphite, typically containing carbon content around 85% to 99%, is widely used in conventional industries like batteries, brake linings, and lubricants where ultrahigh purity is not critical. The refinement process and resultant impurity levels define their suitability for specific applications, with high purity graphite tailored for precision and performance-sensitive environments.
Defining High Purity Graphite: Grades and Specifications
High purity graphite is classified by purity levels exceeding 99.9%, essential for applications requiring minimal impurities such as semiconductor manufacturing, lithium-ion battery anodes, and nuclear reactors. Standard purity graphite generally ranges from 85% to 99% purity, suitable for industrial uses like refractory materials and electrodes where trace contaminants have less impact. Detailed specifications for high purity graphite include low ash content, controlled particle size, and consistent crystalline structure to ensure optimal performance in advanced technological applications.
What Constitutes Standard Purity Graphite?
Standard purity graphite typically contains 95% to 99% carbon content, with impurities such as ash, sulfur, and moisture present in varying amounts. It is produced through mechanical or chemical processes that do not eliminate all contaminants, resulting in lower performance characteristics compared to high purity graphite. This grade is commonly used in industrial applications where ultra-high purity is not essential, including refractories, electrodes, and lubricants.
Key Differences in Production Processes
High purity graphite undergoes rigorous purification methods such as chemical vapor deposition and acid leaching to eliminate impurities, achieving purity levels above 99.99%, whereas standard purity graphite typically relies on mechanical crushing and milling with purity around 95-98%. The production of high purity graphite involves controlled environments and advanced refining techniques to ensure minimal contamination, contrasting with the simpler, less precise processes used for standard grades. These differences significantly impact the material's performance in applications requiring exceptional electrical conductivity and chemical stability.
Comparative Chemical and Physical Properties
High purity graphite typically contains over 99.9% carbon, significantly reducing impurities such as sulfur, ash, and metals found in standard purity graphite, which often ranges from 85% to 99% carbon content. This higher purity results in superior electrical and thermal conductivity, increased corrosion resistance, and enhanced mechanical strength under extreme conditions. Standard purity graphite, with higher impurity levels, exhibits lower thermal stability and electrical performance, making it less suitable for applications requiring precise chemical and physical consistency.
Performance in Industrial Applications
High purity graphite exhibits superior thermal conductivity and electrical performance compared to standard purity graphite, making it ideal for demanding industrial applications such as semiconductors and aerospace components. Its low impurity levels reduce contamination risks, enhancing durability and chemical resistance in harsh environments. Standard purity graphite, while cost-effective, often lacks the consistency and reliability necessary for high-precision tasks where performance directly impacts product quality and operational efficiency.
Cost Implications and Economic Considerations
High purity graphite commands significantly higher prices than standard purity graphite due to its rigorous refining processes and superior performance in critical applications such as semiconductors and nuclear reactors. The increased manufacturing costs for high purity graphite impact product pricing, making it less economically feasible for bulk industrial uses where standard purity graphite suffices. Companies must weigh these cost implications against performance requirements to optimize material selection for financial and operational efficiency.
Purity Impact on Electrical and Thermal Conductivity
High purity graphite typically contains over 99.9% carbon, significantly enhancing its electrical and thermal conductivity compared to standard purity graphite, which may contain impurities such as ash or metal oxides that disrupt electron flow. The reduced impurity levels in high purity graphite minimize scattering of charge carriers and phonons, resulting in superior conductivity performance essential for applications in electronics and heat management. Standard purity graphite often exhibits lower conductivity due to these impurities, limiting its effectiveness in high-performance electrical and thermal systems.
Environmental and Safety Aspects
High purity graphite offers superior environmental benefits due to its reduced impurity levels, minimizing toxic emissions during manufacturing and application compared to standard purity graphite. The enhanced chemical stability of high purity graphite significantly lowers the risk of hazardous contamination and improves safety protocols in industrial settings. Standard purity graphite, with higher impurity content, poses increased environmental challenges and safety hazards related to byproduct disposal and airborne particulates.
Selection Criteria: How to Choose the Right Graphite Purity
Selecting the right graphite purity hinges on the specific application requirements, where high purity graphite, typically above 99.9% carbon content, is essential for electronics, semiconductors, and nuclear industries due to its low impurity levels and superior electrical conductivity. Standard purity graphite, with carbon content ranging from 85% to 99%, suits general industrial uses such as refractory materials and lubricants, offering cost-effectiveness without stringent purity demands. Key selection criteria include thermal stability, electrical conductivity, contamination tolerance, and budget considerations, ensuring optimal performance and longevity in the intended application.
High Purity Graphite vs Standard Purity Graphite Infographic
 materialdif.com
materialdif.com