Spherical graphite exhibits a uniform, rounded particle shape that enhances its conductivity and packing density, making it ideal for use in lithium-ion battery anodes. Crystalline graphite, characterized by its layered, planar structure, offers high electrical conductivity and thermal stability but lacks the optimized morphology for efficient energy storage. Choosing spherical graphite over crystalline graphite can significantly improve battery performance due to better electrochemical properties and structural integrity.
Table of Comparison
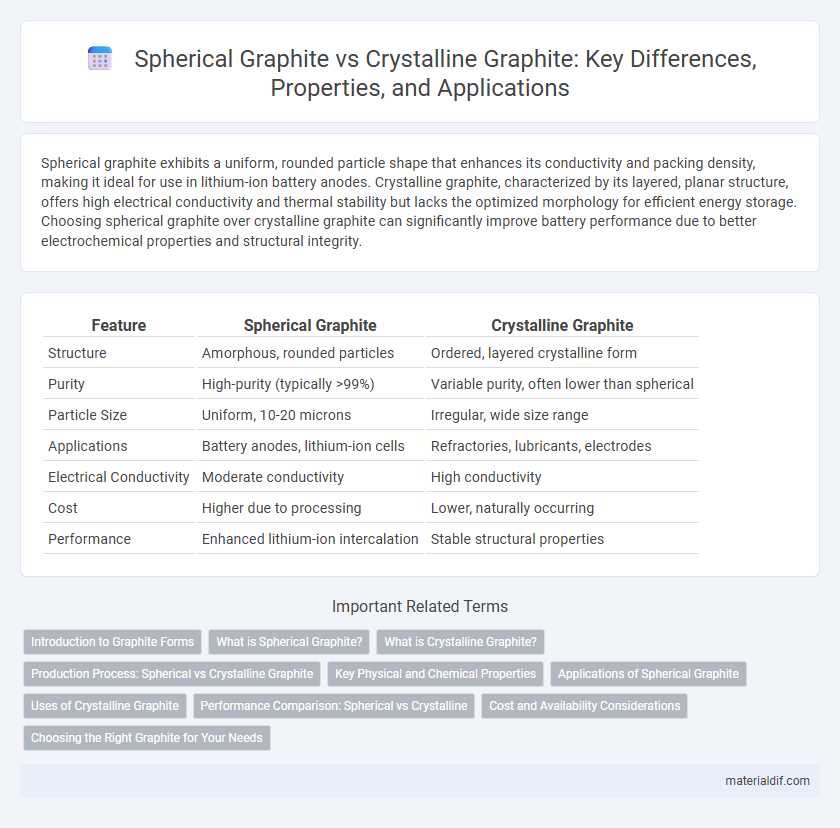
| Feature | Spherical Graphite | Crystalline Graphite |
|---|---|---|
| Structure | Amorphous, rounded particles | Ordered, layered crystalline form |
| Purity | High-purity (typically >99%) | Variable purity, often lower than spherical |
| Particle Size | Uniform, 10-20 microns | Irregular, wide size range |
| Applications | Battery anodes, lithium-ion cells | Refractories, lubricants, electrodes |
| Electrical Conductivity | Moderate conductivity | High conductivity |
| Cost | Higher due to processing | Lower, naturally occurring |
| Performance | Enhanced lithium-ion intercalation | Stable structural properties |
Introduction to Graphite Forms
Spherical graphite and crystalline graphite represent key forms of natural and synthetic graphite used in various industrial applications, notably in lithium-ion battery anodes. Spherical graphite is characterized by its near-perfect round shape and high purity, enhancing battery performance through improved conductivity and cycle life. Crystalline graphite, distinguished by its layered planar structure and varying crystallite sizes, offers different electrical and thermal properties suitable for lubricants, refractories, and electrodes.
What is Spherical Graphite?
Spherical graphite is a form of synthetic graphite characterized by its rounded, spherical particle shape that enhances packing density and conductivity. Unlike crystalline graphite, which has a flaky, layered structure, spherical graphite undergoes a processing technique called spheroidization to improve its electrochemical performance in lithium-ion battery anodes. This shape transformation increases surface area uniformity and improves lithium-ion intercalation, making spherical graphite a critical material in advanced battery technologies.
What is Crystalline Graphite?
Crystalline graphite is a form of graphite characterized by its highly ordered and tightly packed carbon atoms arranged in a hexagonal lattice structure, which provides excellent electrical conductivity and thermal stability. Unlike spherical graphite, which has a more rounded shape suited for battery anodes, crystalline graphite features flat, layered sheets that contribute to its mechanical strength and lubricating properties. Its purity and crystallinity make it essential in industrial applications such as electrodes, refractories, and high-performance batteries.
Production Process: Spherical vs Crystalline Graphite
Spherical graphite production involves pulverizing flaky graphite into fine particles followed by extensive shaping and purification processes to create uniform, rounded particles ideal for battery anodes. Crystalline graphite is typically obtained through mining and mechanical exfoliation, maintaining its layered structure with distinctive plate-like crystals. The manufacturing complexity and precision in spherical graphite processes directly impact its electrochemical performance compared to the naturally occurring crystalline form.
Key Physical and Chemical Properties
Spherical graphite exhibits a high degree of purity with particle sizes typically ranging from 10 to 20 microns, optimized for lithium-ion battery anodes due to excellent electrical conductivity and cycling stability. Crystalline graphite has a well-ordered layered structure with larger particle sizes and higher crystallinity, resulting in superior thermal conductivity and mechanical strength. The key physical differences lie in morphology and size, while chemically, spherical graphite typically contains fewer impurities, enhancing its electrochemical performance compared to crystalline graphite.
Applications of Spherical Graphite
Spherical graphite is predominantly used as an anode material in lithium-ion batteries due to its high purity, excellent conductivity, and uniform particle size, which enhances battery capacity and cycle life. Its spherical shape improves electrode packing density and reduces expansion during charge cycles, making it ideal for electric vehicles and portable electronics. In contrast, crystalline graphite, with its flaky structure, is more suited for applications in refractory materials and lubricants where thermal resistance is critical.
Uses of Crystalline Graphite
Crystalline graphite, characterized by its ordered atomic structure, is primarily used in high-temperature applications such as refractory materials, electrodes in electric arc furnaces, and nuclear reactors due to its excellent thermal conductivity and stability. It also plays a critical role in the production of lubricants, batteries, and brake linings, where its layered structure provides superior lubricity and electrical conductivity. Unlike spherical graphite, which is predominantly used in lithium-ion battery anodes, crystalline graphite's diverse industrial applications leverage its structural integrity and thermal properties.
Performance Comparison: Spherical vs Crystalline
Spherical graphite offers significantly higher electrochemical performance compared to crystalline graphite due to its uniform particle size and enhanced conductivity, making it ideal for lithium-ion battery anodes. Crystalline graphite exhibits superior structural stability but lower rate capabilities, limiting its efficiency in high-power applications. The optimized morphology of spherical graphite results in better cycle life and faster charge-discharge rates, providing a critical advantage in battery performance.
Cost and Availability Considerations
Spherical graphite generally incurs higher production costs due to complex processing methods like milling and rounding, whereas crystalline graphite is cheaper to produce owing to simpler mining and purification techniques. Availability of spherical graphite is limited by the need for high-purity natural flake graphite feedstock and specialized manufacturing facilities, while crystalline graphite benefits from more abundant natural deposits and established mining infrastructure. Cost-efficiency and supply chain stability often make crystalline graphite the preferred choice for bulk industrial applications, despite spherical graphite's superior performance in battery anodes.
Choosing the Right Graphite for Your Needs
Spherical graphite offers superior conductivity and is highly favored in lithium-ion battery anodes due to its uniform particle size and excellent packing density. Crystalline graphite, with its layered structure, provides higher thermal stability and is widely used in industrial applications such as refractory materials and lubricants. Selecting the right graphite depends on application requirements, balancing factors like electrical performance, thermal resistance, and mechanical strength.
Spherical Graphite vs Crystalline Graphite Infographic
 materialdif.com
materialdif.com