Amorphous graphite consists of small, irregularly arranged carbon flakes, offering excellent lubricating properties suitable for industrial applications like brake linings and pencils. Crystalline graphite features a well-ordered, layered structure with high thermal conductivity and electrical conductivity, ideal for high-performance uses such as electrodes and battery anodes. Understanding the differences in structure and properties between amorphous and crystalline graphite helps optimize material selection for specific graphite pet products.
Table of Comparison
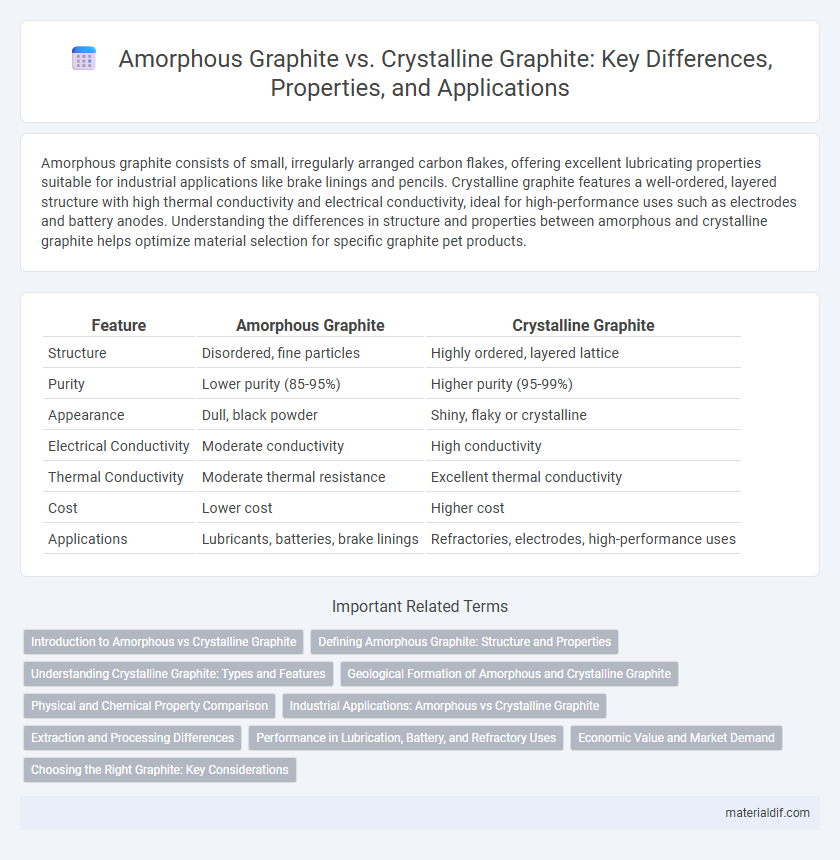
| Feature | Amorphous Graphite | Crystalline Graphite |
|---|---|---|
| Structure | Disordered, fine particles | Highly ordered, layered lattice |
| Purity | Lower purity (85-95%) | Higher purity (95-99%) |
| Appearance | Dull, black powder | Shiny, flaky or crystalline |
| Electrical Conductivity | Moderate conductivity | High conductivity |
| Thermal Conductivity | Moderate thermal resistance | Excellent thermal conductivity |
| Cost | Lower cost | Higher cost |
| Applications | Lubricants, batteries, brake linings | Refractories, electrodes, high-performance uses |
Introduction to Amorphous vs Crystalline Graphite
Amorphous graphite consists of small, randomly oriented graphite crystals with a high degree of disorder, resulting in lower electrical conductivity and mechanical strength compared to crystalline graphite. Crystalline graphite features well-ordered, hexagonal layers of carbon atoms arranged in a lattice, providing superior thermal conductivity, electrical properties, and structural integrity. The distinction between amorphous and crystalline graphite is critical in applications such as battery anodes, lubricants, and refractories, where performance depends on the atomic arrangement and purity of the graphite material.
Defining Amorphous Graphite: Structure and Properties
Amorphous graphite consists of disordered, randomly oriented carbon layers lacking the well-defined hexagonal lattice found in crystalline graphite, resulting in less electrical conductivity and lower thermal stability. Its structure is characterized by small, turbostratic microcrystals with high defect density, contributing to its relatively low purity and softness compared to crystalline forms. These properties make amorphous graphite suitable for applications such as battery anodes and lubricants, where cost-effectiveness outweighs the need for superior conductivity or mechanical strength.
Understanding Crystalline Graphite: Types and Features
Crystalline graphite is characterized by its highly ordered atomic structure, consisting of layers of hexagonally arranged carbon atoms. The two primary types of crystalline graphite are natural graphite, found in metamorphic rocks, and synthetic graphite, produced through high-temperature treatment of carbon-rich materials. Its features include excellent electrical conductivity, high thermal stability, and anisotropic properties due to the crystal lattice orientation.
Geological Formation of Amorphous and Crystalline Graphite
Amorphous graphite forms primarily from fine-grained organic material subjected to low-grade metamorphism, resulting in disordered carbon structures commonly found in sedimentary rocks and low-temperature geological environments. Crystalline graphite develops through high-grade metamorphic processes, where intense heat and pressure reorganize carbon atoms into well-ordered hexagonal crystal lattices, often associated with schists and gneisses in deep crustal settings. The geological formation differences influence their structural properties, with amorphous graphite exhibiting lower crystallinity and crystalline graphite showing highly ordered graphitic layers.
Physical and Chemical Property Comparison
Amorphous graphite is characterized by a disordered structure with small, randomly oriented graphite flakes, resulting in lower density and hardness compared to crystalline graphite, which has a well-ordered hexagonal lattice providing higher thermal conductivity and electrical conductivity. Chemically, amorphous graphite exhibits higher reactivity and impurity content due to its irregular structure, while crystalline graphite demonstrates greater chemical stability and resistance to oxidation. These physical and chemical differences influence their applications, with amorphous graphite favored for lubricants and refractory materials, and crystalline graphite preferred in high-performance electrodes and battery anodes.
Industrial Applications: Amorphous vs Crystalline Graphite
Amorphous graphite, characterized by its fine, randomly oriented flakes, excels in applications requiring high lubricity and low-cost raw materials, commonly used in brake linings, pencils, and foundry facings. Crystalline graphite, with a well-ordered layered structure, offers superior electrical conductivity and thermal resistance, making it ideal for electrodes in electric arc furnaces, battery anodes, and high-performance lubricants. The distinct physical properties between amorphous and crystalline graphite determine their industrial roles, optimizing product performance and cost-efficiency across diverse sectors.
Extraction and Processing Differences
Amorphous graphite is primarily extracted via surface mining methods due to its occurrence in fine, flaky deposits, whereas crystalline graphite is often obtained through underground mining targeting larger, more defined vein formations. Processing amorphous graphite typically involves flotation and fine grinding to enhance purity, while crystalline graphite requires advanced thermal purification and sometimes chemical treatments to achieve higher crystalline quality. The distinct structural differences between amorphous and crystalline graphite directly influence their extraction techniques and refining processes, impacting overall yield and product grade.
Performance in Lubrication, Battery, and Refractory Uses
Amorphous graphite exhibits superior lubrication performance due to its smaller particle size and higher surface area, enabling better film formation and reduced friction in mechanical systems. Crystalline graphite outperforms amorphous types in battery applications, particularly in lithium-ion anodes, because of its well-ordered layered structure that facilitates efficient lithium-ion intercalation and enhanced electrical conductivity. In refractory uses, crystalline graphite's higher purity and thermal stability contribute to improved resistance to thermal shock and chemical attack, whereas amorphous graphite is less durable under extreme high-temperature conditions.
Economic Value and Market Demand
Amorphous graphite, characterized by its fine particle size and lower purity, commands a lower economic value but sees significant demand in applications like brake linings, lubricants, and batteries due to cost efficiency. Crystalline graphite, including flake and vein types, exhibits higher purity and superior conductivity, resulting in a higher market price and robust demand in high-tech industries such as lithium-ion batteries, refractory materials, and fuel cells. Market trends indicate increasing investment in crystalline graphite production driven by the expanding electric vehicle sector, elevating its economic value compared to amorphous graphite.
Choosing the Right Graphite: Key Considerations
Choosing the right graphite involves understanding the structural differences between amorphous and crystalline graphite, which affect their conductivity and thermal stability. Amorphous graphite features small, disordered carbon particles ideal for lubricants and pencils, while crystalline graphite offers well-ordered layers with superior electrical conductivity and mechanical strength, essential for batteries and industrial applications. Key considerations include the specific application requirements, such as purity, particle size, and environmental resistance, to ensure optimal performance and cost-efficiency.
Amorphous Graphite vs Crystalline Graphite Infographic
 materialdif.com
materialdif.com