Graphite crucibles offer superior thermal conductivity and resistance to thermal shock compared to clay crucibles, making them ideal for high-temperature metal melting applications. Clay crucibles, while more affordable and chemically inert, tend to be more fragile and less durable under intense heat cycles. Choosing between graphite and clay crucibles depends on the specific requirements of thermal efficiency, durability, and cost-effectiveness in metalworking processes.
Table of Comparison
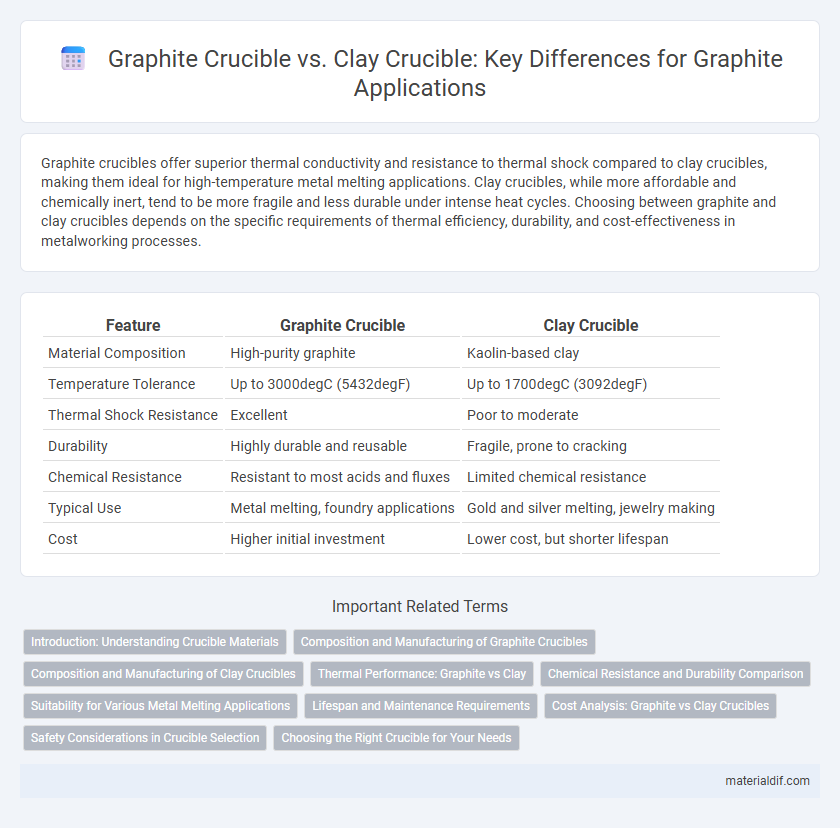
| Feature | Graphite Crucible | Clay Crucible |
|---|---|---|
| Material Composition | High-purity graphite | Kaolin-based clay |
| Temperature Tolerance | Up to 3000degC (5432degF) | Up to 1700degC (3092degF) |
| Thermal Shock Resistance | Excellent | Poor to moderate |
| Durability | Highly durable and reusable | Fragile, prone to cracking |
| Chemical Resistance | Resistant to most acids and fluxes | Limited chemical resistance |
| Typical Use | Metal melting, foundry applications | Gold and silver melting, jewelry making |
| Cost | Higher initial investment | Lower cost, but shorter lifespan |
Introduction: Understanding Crucible Materials
Graphite crucibles offer superior thermal conductivity and resistance to thermal shock compared to clay crucibles, making them ideal for high-temperature metal melting and casting processes. Clay crucibles, composed primarily of refractory clay and sometimes reinforced with carbon, provide excellent resistance to chemical corrosion but have lower thermal conductivity, resulting in slower heating times. Selecting between graphite and clay crucibles depends on application temperature requirements, chemical compatibility, and mechanical durability needed for specific metallurgical tasks.
Composition and Manufacturing of Graphite Crucibles
Graphite crucibles are primarily composed of high-purity graphite mixed with bonding agents such as clay or pitch, providing excellent thermal conductivity and resistance to thermal shock. Their manufacturing involves a high-temperature baking process that carbonizes the binder, resulting in a crucible with superior durability and stability at extreme temperatures compared to clay crucibles. This composition and manufacturing method enable graphite crucibles to withstand rapid temperature changes and offer enhanced resistance to chemical corrosion during metal melting processes.
Composition and Manufacturing of Clay Crucibles
Clay crucibles are primarily composed of refractory clay materials such as kaolin, ball clay, and fireclay, combined with organic binders to enhance plasticity and strength. Their manufacturing involves molding the clay mixture into the desired shape, followed by a controlled drying process and high-temperature firing to achieve durability and thermal stability. Unlike graphite crucibles, which are made from carbon-based materials offering superior thermal conductivity, clay crucibles exhibit lower thermal shock resistance but are cost-effective and suitable for less demanding melting applications.
Thermal Performance: Graphite vs Clay
Graphite crucibles exhibit superior thermal conductivity and can withstand rapid temperature changes without cracking, making them ideal for high-temperature metal melting applications. Clay crucibles offer lower thermal conductivity and slower heat transfer, which results in longer heating times and a higher risk of thermal shock under sudden temperature fluctuations. The enhanced thermal stability of graphite crucibles improves efficiency and durability in industrial processes compared to clay alternatives.
Chemical Resistance and Durability Comparison
Graphite crucibles exhibit superior chemical resistance against molten metals and aggressive fluxes compared to clay crucibles, which are more susceptible to corrosion and contamination. The durability of graphite crucibles stands out due to their thermal shock resistance and ability to withstand repeated high-temperature cycles without significant wear. Clay crucibles, while cost-effective, tend to degrade faster under intense thermal and chemical stress, limiting their lifespan in industrial applications.
Suitability for Various Metal Melting Applications
Graphite crucibles offer superior thermal conductivity and resistance to thermal shock, making them ideal for melting metals like silver, gold, and aluminum at high temperatures. Clay crucibles excel in melting ferrous metals and alloys due to their durability and ability to withstand prolonged exposure to oxidizing environments. Selecting the appropriate crucible depends on the specific melting temperature, metal type, and required chemical resistance for optimal performance in metal casting applications.
Lifespan and Maintenance Requirements
Graphite crucibles typically offer a longer lifespan than clay crucibles due to their superior thermal conductivity and resistance to thermal shock, which reduces the risk of cracking during rapid temperature changes. Maintenance for graphite crucibles is generally simpler because they are less prone to erosion and chemical reactions with molten metals, while clay crucibles require more frequent inspections and careful handling to prevent breakage and contamination. As a result, industries that demand high durability and lower maintenance costs often prefer graphite crucibles for extended use in metal melting processes.
Cost Analysis: Graphite vs Clay Crucibles
Graphite crucibles generally offer a higher initial cost compared to clay crucibles but provide superior thermal conductivity and longer lifespan, reducing overall operational expenses. Clay crucibles are more affordable upfront but tend to degrade faster under high temperatures, leading to frequent replacements and increased long-term costs. Evaluating total cost of ownership, graphite crucibles prove more cost-effective for industrial melting applications despite their higher purchase price.
Safety Considerations in Crucible Selection
Graphite crucibles offer superior thermal shock resistance and chemical stability, reducing the risk of cracking and contamination during high-temperature metal melting. Clay crucibles, while more affordable, tend to have lower mechanical strength and are more susceptible to thermal stress, increasing the likelihood of breakage and potential safety hazards. Selecting graphite crucibles enhances safety by minimizing exposure to molten metal spills and ensuring consistent performance under extreme heat conditions.
Choosing the Right Crucible for Your Needs
Graphite crucibles offer excellent thermal conductivity and resistance to thermal shock, making them ideal for high-temperature metal melting and casting applications. Clay crucibles, while more affordable and widely available, are better suited for lower-temperature processes and non-corrosive materials due to their brittleness and lower thermal conductivity. Selecting the right crucible depends on factors such as melting point requirements, material compatibility, and budget constraints to ensure efficiency and durability in your specific use case.
Graphite Crucible vs Clay Crucible Infographic
 materialdif.com
materialdif.com