Zinc oxide and zinc stearate serve distinct purposes in pet products, with zinc oxide commonly used for its skin-protective and antimicrobial properties, helping to soothe irritation and promote healing. Zinc stearate acts as a lubricant and release agent, improving the texture and application of topical treatments without providing direct skin benefits. Understanding their differences helps pet owners select the right ingredient for effective skin care and grooming formulations.
Table of Comparison
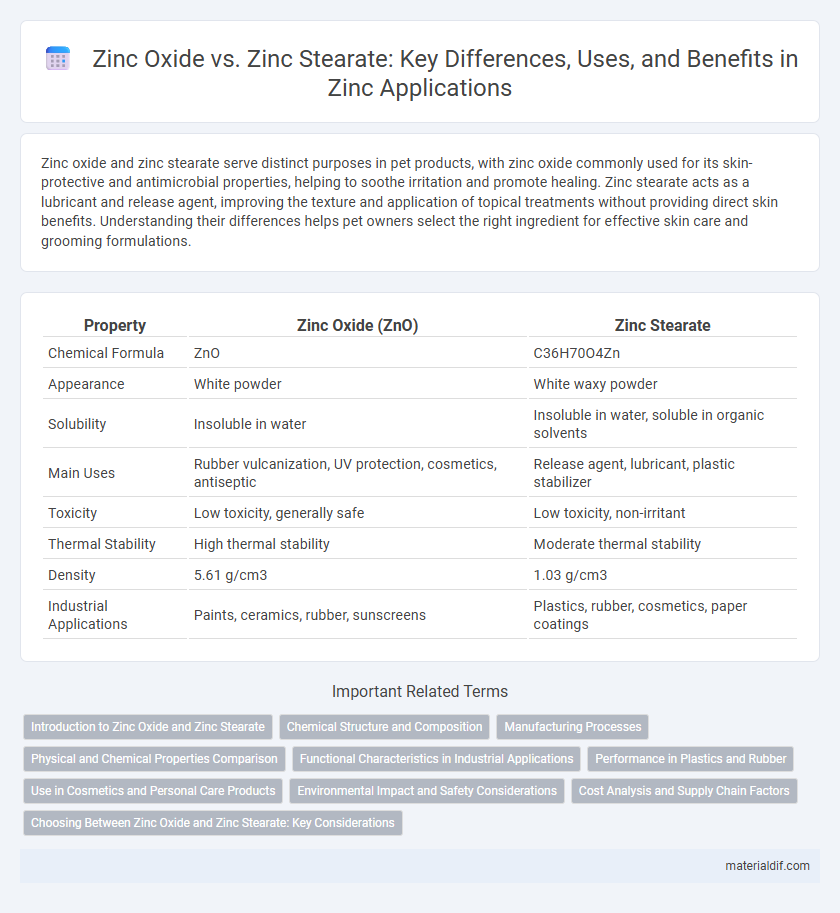
| Property | Zinc Oxide (ZnO) | Zinc Stearate |
|---|---|---|
| Chemical Formula | ZnO | C36H70O4Zn |
| Appearance | White powder | White waxy powder |
| Solubility | Insoluble in water | Insoluble in water, soluble in organic solvents |
| Main Uses | Rubber vulcanization, UV protection, cosmetics, antiseptic | Release agent, lubricant, plastic stabilizer |
| Toxicity | Low toxicity, generally safe | Low toxicity, non-irritant |
| Thermal Stability | High thermal stability | Moderate thermal stability |
| Density | 5.61 g/cm3 | 1.03 g/cm3 |
| Industrial Applications | Paints, ceramics, rubber, sunscreens | Plastics, rubber, cosmetics, paper coatings |
Introduction to Zinc Oxide and Zinc Stearate
Zinc oxide is an inorganic compound widely used in cosmetics, pharmaceuticals, and rubber manufacturing due to its antimicrobial and UV-blocking properties. Zinc stearate, a zinc salt of stearic acid, serves primarily as a lubricant and release agent in plastics, rubber, and cosmetics industries. Both compounds offer unique functional benefits, with zinc oxide emphasizing protective and therapeutic roles, while zinc stearate enhances processing efficiency and product texture.
Chemical Structure and Composition
Zinc oxide (ZnO) is an inorganic compound composed of zinc and oxygen atoms in a crystalline lattice, characterized by its stable, non-metallic oxide structure. Zinc stearate, a zinc salt of stearic acid, consists of zinc ions coordinated with long-chain fatty acid anions, forming an organic coordination complex with hydrophobic properties. The fundamental difference lies in zinc oxide's oxide framework versus zinc stearate's metal-organic composition, influencing their solubility, thermal stability, and application spectrum.
Manufacturing Processes
Zinc oxide is produced primarily through the French process, which involves vapor-phase oxidation of metallic zinc at high temperatures, resulting in fine, white, powdery particles ideal for use in rubber, cosmetics, and pharmaceuticals. Zinc stearate is synthesized via a chemical reaction between zinc salts, such as zinc sulfate or zinc chloride, and stearic acid, typically in a hot aqueous solution followed by filtration and drying, yielding a hydrophobic, lubricating powder commonly used in plastics, cosmetics, and rubber processing. The distinct manufacturing methods reflect their different functional applications, with zinc oxide relying on thermal oxidation and zinc stearate on chemical precipitation.
Physical and Chemical Properties Comparison
Zinc oxide is an inorganic compound with a white powder form, characterized by high thermal stability, low solubility in water, and strong UV absorption properties, making it ideal for applications in sunscreens and rubber vulcanization. Zinc stearate, an organic compound composed of zinc ions and stearate ligands, appears as a waxy solid with hydrophobic properties, low melting point around 120degC, and good lubricating and water-repellent characteristics, commonly used as a release agent and in plastics. Chemically, zinc oxide acts as an amphoteric oxide reacting with both acids and bases, while zinc stearate is a zinc salt of a fatty acid, exhibiting stability in organic solvents but hydrolyzing in aqueous acidic or alkaline environments.
Functional Characteristics in Industrial Applications
Zinc oxide exhibits excellent antimicrobial, UV-blocking, and catalytic properties, making it indispensable in rubber manufacturing, cosmetics, and pharmaceuticals for enhancing durability and protection. Zinc stearate, characterized by its lubricating, water-resistant, and insulating features, is widely used as a release agent, lubricant, and thickener in plastics, rubber, and paints. The distinct functional characteristics of zinc oxide and zinc stearate dictate their specialized industrial applications based on performance requirements.
Performance in Plastics and Rubber
Zinc oxide offers superior antimicrobial and UV protection properties in plastics and rubber, enhancing durability and extending product lifespan under harsh conditions. Zinc stearate acts primarily as a lubricant and release agent, improving processing efficiency and surface finish but providing limited protective benefits. In performance-critical applications, zinc oxide is preferred for its multifunctional roles, while zinc stearate is chosen for manufacturing ease and mold release.
Use in Cosmetics and Personal Care Products
Zinc oxide is widely used in cosmetics and personal care products for its excellent UV protection and skin-soothing properties, making it a key ingredient in sunscreens, diaper rash creams, and mineral makeup. Zinc stearate primarily functions as a lubricant and anti-caking agent in cosmetic powders, enhancing texture and application without offering significant skin benefits or UV protection. Both compounds contribute to product stability, but zinc oxide's role in sun protection and skin health distinguishes it within formulations.
Environmental Impact and Safety Considerations
Zinc oxide is widely utilized in sunscreens and rubber manufacturing due to its non-toxic nature and lower environmental impact, as it is less likely to bioaccumulate and degrade safely in ecosystems. Zinc stearate, commonly used as a lubricant and release agent, poses higher risks of environmental persistence and potential toxic effects on aquatic life because of its fatty acid derivatives. Safety considerations favor zinc oxide for human exposure, while zinc stearate requires careful handling to minimize dust inhalation and reduce environmental contamination.
Cost Analysis and Supply Chain Factors
Zinc oxide typically incurs lower costs due to its wide availability and simpler processing methods compared to zinc stearate, which demands more complex manufacturing processes involving fatty acids. Supply chain factors favor zinc oxide as it benefits from established global mining operations and bulk production, ensuring steady availability and pricing stability. In contrast, zinc stearate relies on both zinc and stearic acid supply chains, introducing potential variability and higher logistical expenses.
Choosing Between Zinc Oxide and Zinc Stearate: Key Considerations
Zinc oxide is widely used for its antimicrobial and UV-blocking properties, making it ideal for skincare and sunscreen products, while zinc stearate serves primarily as a lubricant and mold release agent in pharmaceutical and cosmetic manufacturing. When choosing between zinc oxide and zinc stearate, consider the required functional benefits: zinc oxide offers protection and healing, whereas zinc stearate provides improved texture and manufacturing efficiency. Additionally, factors such as intended application, solubility, and regulatory compliance should guide the selection to ensure optimal performance.
Zinc oxide vs zinc stearate Infographic
 materialdif.com
materialdif.com