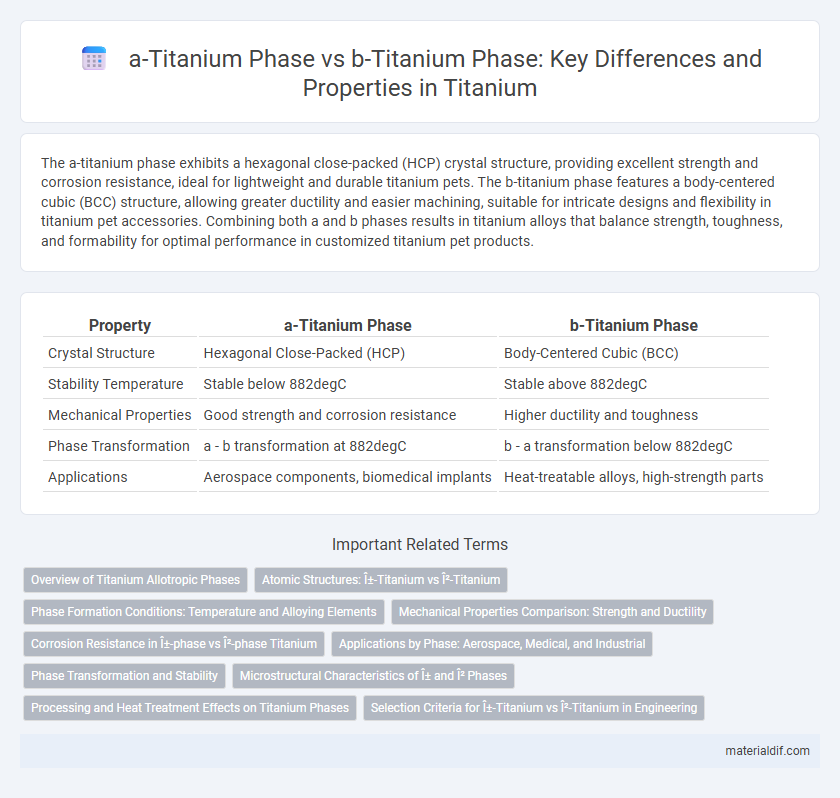
The a-titanium phase exhibits a hexagonal close-packed (HCP) crystal structure, providing excellent strength and corrosion resistance, ideal for lightweight and durable titanium pets. The b-titanium phase features a body-centered cubic (BCC) structure, allowing greater ductility and easier machining, suitable for intricate designs and flexibility in titanium pet accessories. Combining both a and b phases results in titanium alloys that balance strength, toughness, and formability for optimal performance in customized titanium pet products.
Table of Comparison
| Property | a-Titanium Phase | b-Titanium Phase |
|---|---|---|
| Crystal Structure | Hexagonal Close-Packed (HCP) | Body-Centered Cubic (BCC) |
| Stability Temperature | Stable below 882degC | Stable above 882degC |
| Mechanical Properties | Good strength and corrosion resistance | Higher ductility and toughness |
| Phase Transformation | a - b transformation at 882degC | b - a transformation below 882degC |
| Applications | Aerospace components, biomedical implants | Heat-treatable alloys, high-strength parts |
Overview of Titanium Allotropic Phases
Titanium exhibits two primary allotropic phases: the a-phase, which is hexagonal close-packed (HCP) and stable at lower temperatures up to 882degC, and the b-phase, characterized by a body-centered cubic (BCC) structure stable above 882degC. The a-phase provides excellent corrosion resistance and good strength, making it ideal for aerospace and biomedical applications, while the b-phase allows for enhanced formability and is often alloyed to improve mechanical properties. Understanding the temperature-dependent phase transformation between a-Titanium and b-Titanium is crucial for optimizing heat treatment processes and tailoring alloy performance.
Atomic Structures: α-Titanium vs β-Titanium
a-Titanium exhibits a hexagonal close-packed (HCP) atomic structure, characterized by high strength and excellent corrosion resistance ideal for aerospace applications. In contrast, b-Titanium possesses a body-centered cubic (BCC) structure, offering enhanced ductility and formability suitable for complex shapes and high-temperature environments. The atomic arrangement differences directly influence mechanical properties and phase stability in titanium alloys.
Phase Formation Conditions: Temperature and Alloying Elements
a-Titanium phase forms at lower temperatures below 882degC and is stabilized by elements such as aluminum, oxygen, and nitrogen, which increase the phase's strength and thermal stability. b-Titanium phase appears at temperatures above 882degC and is stabilized by beta stabilizers like vanadium, molybdenum, and iron, which lower the transformation temperature and enhance ductility. The precise phase formation depends on the titanium alloy's composition and thermal history, influencing mechanical properties and application suitability.
Mechanical Properties Comparison: Strength and Ductility
a-Titanium phase exhibits higher strength and better creep resistance due to its hexagonal close-packed (HCP) crystal structure, whereas b-Titanium phase, with its body-centered cubic (BCC) lattice, offers enhanced ductility and toughness. The b-phase's ability to deform more easily under stress makes it preferable for applications requiring significant formability. Mechanical properties of titanium alloys can be tailored by controlling the balance between a and b phases through heat treatment and alloying elements like aluminum and vanadium.
Corrosion Resistance in α-phase vs β-phase Titanium
a-Titanium phase exhibits superior corrosion resistance compared to b-Titanium phase due to its hexagonal close-packed (HCP) crystal structure, which provides a more stable and less reactive surface in aggressive environments. b-Titanium phase, with its body-centered cubic (BCC) structure, tends to have higher diffusion rates and more grain boundary activity, making it more susceptible to localized corrosion and oxidation. This difference in crystal structure strongly influences the formation and integrity of the passive oxide layer, crucial for titanium's overall corrosion resistance.
Applications by Phase: Aerospace, Medical, and Industrial
a-Titanium phase exhibits excellent corrosion resistance and high strength at elevated temperatures, making it ideal for aerospace components like airframes and engine parts. b-Titanium phase offers superior formability and higher strength after heat treatment, which suits medical implants such as orthopedic devices and dental implants due to biocompatibility and fatigue resistance. Industrial applications leverage a+b titanium alloys to balance mechanical properties and manufacturability for tools, automotive parts, and chemical processing equipment.
Phase Transformation and Stability
The a-titanium phase exhibits a hexagonal close-packed (HCP) crystal structure, which remains stable at lower temperatures and provides excellent corrosion resistance and strength. The b-titanium phase has a body-centered cubic (BCC) crystal structure, stable at higher temperatures and known for its high ductility and formability. Phase transformation between a and b phases occurs around 882degC, with the addition of alloying elements like vanadium and molybdenum enhancing the b-phase stability at room temperature.
Microstructural Characteristics of α and β Phases
The a-Titanium phase exhibits a hexagonal close-packed (HCP) microstructure characterized by limited slip systems, which confers high strength and excellent corrosion resistance. In contrast, the b-Titanium phase has a body-centered cubic (BCC) structure that enhances ductility and toughness due to its more symmetrical atomic arrangement and increased slip systems. Microstructural differences between a and b phases significantly influence mechanical properties, with a-phase favoring high-temperature stability and b-phase allowing easier deformation and phase transformations.
Processing and Heat Treatment Effects on Titanium Phases
The a-titanium phase exhibits a hexagonal close-packed (HCP) structure that provides excellent corrosion resistance and good strength, commonly stabilized through annealing and slow cooling procedures. The b-titanium phase, characterized by a body-centered cubic (BCC) structure, forms at higher temperatures and can be stabilized at room temperature via rapid quenching or alloying with beta stabilizers such as vanadium or molybdenum. Heat treatment techniques like solution treatment and aging allow precise control over the microstructure, enabling phase transformation that enhances mechanical properties and tailors titanium alloys for aerospace and biomedical applications.
Selection Criteria for α-Titanium vs β-Titanium in Engineering
a-Titanium exhibits a hexagonal close-packed (HCP) crystal structure that provides excellent corrosion resistance and high strength at room temperature, making it ideal for aerospace and medical implants. b-Titanium has a body-centered cubic (BCC) structure that enables superior formability and higher toughness at elevated temperatures, favored in automotive and industrial applications. Selection criteria hinge on operating temperature, mechanical properties, and manufacturability requirements, with a-Ti suited for stability and corrosion resistance and b-Ti preferred for ductility and heat treatment potential.
α-Titanium Phase vs β-Titanium Phase Infographic
 materialdif.com
materialdif.com