Tin-plating provides a smooth, corrosion-resistant coating primarily used for protecting steel and enhancing solderability in electronic components, whereas galvanizing involves applying a thicker layer of zinc to offer robust rust prevention, particularly for outdoor and heavy-duty applications. Tin-plated surfaces ensure excellent conductivity and an attractive finish but may wear off under abrasive conditions, while galvanized coatings deliver superior durability against weathering and mechanical damage. Choosing between tin-plating and galvanizing depends on the specific environmental exposure, required appearance, and longevity needed for the metal part.
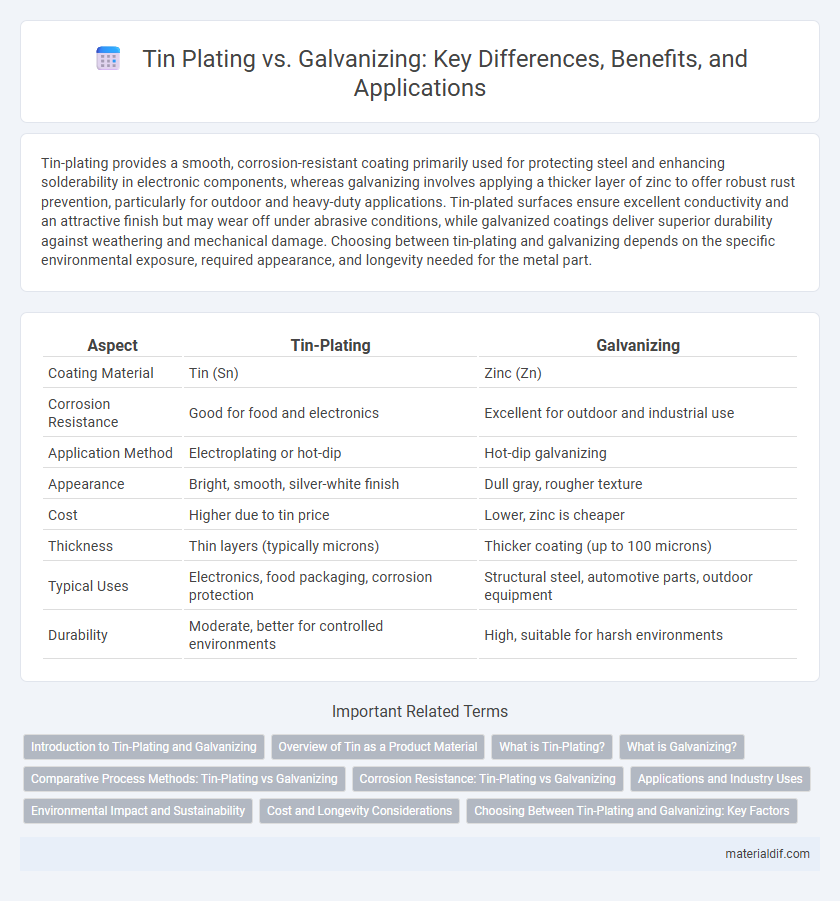
Table of Comparison
| Aspect | Tin-Plating | Galvanizing |
|---|---|---|
| Coating Material | Tin (Sn) | Zinc (Zn) |
| Corrosion Resistance | Good for food and electronics | Excellent for outdoor and industrial use |
| Application Method | Electroplating or hot-dip | Hot-dip galvanizing |
| Appearance | Bright, smooth, silver-white finish | Dull gray, rougher texture |
| Cost | Higher due to tin price | Lower, zinc is cheaper |
| Thickness | Thin layers (typically microns) | Thicker coating (up to 100 microns) |
| Typical Uses | Electronics, food packaging, corrosion protection | Structural steel, automotive parts, outdoor equipment |
| Durability | Moderate, better for controlled environments | High, suitable for harsh environments |
Introduction to Tin-Plating and Galvanizing
Tin-plating involves coating a metal surface with a thin layer of tin to enhance corrosion resistance and improve solderability, commonly used in electronic components and food packaging. Galvanizing refers to applying a protective zinc coating to steel or iron to prevent rusting, often employed in construction and automotive industries. Both methods extend metal durability but differ in coating materials and specific applications tailored to environmental exposure and mechanical requirements.
Overview of Tin as a Product Material
Tin is a corrosion-resistant metal widely used as a coating material in both tin-plating and galvanizing processes to protect steel and other substrates. Tin-plating involves applying a thin layer of pure tin to enhance the surface's solderability, corrosion resistance, and food safety, commonly used in electronics and food packaging. Unlike galvanizing, which relies on zinc for sacrificial protection, tin's non-toxic properties make it ideal for applications requiring direct contact with consumables.
What is Tin-Plating?
Tin-plating involves coating a thin layer of tin onto the surface of metal, typically steel or iron, to enhance corrosion resistance and improve solderability. This electrochemical process creates a smooth, non-toxic, and conductive surface ideal for food packaging, electronics, and hardware applications. Tin-plated metals offer superior protection against rust while maintaining a bright, attractive finish.
What is Galvanizing?
Galvanizing is a metal coating process that applies a protective layer of zinc to steel or iron to prevent corrosion and rust. This zinc coating acts as a sacrificial anode, corroding before the underlying metal, thereby extending the lifespan of the material. Galvanizing is widely used in construction, automotive, and infrastructure industries due to its durability and resistance to environmental damage.
Comparative Process Methods: Tin-Plating vs Galvanizing
Tin-plating involves electrochemically depositing a thin layer of tin onto a metal surface to provide corrosion resistance and solderability, commonly used for electronic components. Galvanizing, typically employing a hot-dip process, coats steel or iron with a thicker zinc layer that offers robust protection against rust and mechanical damage. While tin-plating ensures excellent electrical conductivity and a smooth finish, galvanizing prioritizes durable, long-term corrosion prevention in harsher environmental conditions.
Corrosion Resistance: Tin-Plating vs Galvanizing
Tin-plating offers corrosion resistance by creating a protective layer that prevents oxidation and solderability benefits, especially on steel and copper substrates. Galvanizing provides superior corrosion resistance through a thick zinc coating that sacrifices itself to protect underlying metal from rust, ideal for outdoor or harsh environments. While tin-plating excels in electrical conductivity and aesthetic finishes, galvanizing is preferred for long-term protection against corrosion in industrial and structural applications.
Applications and Industry Uses
Tin-plating is widely used in the electronics industry for its excellent corrosion resistance and solderability, making it ideal for coating electrical components, food cans, and automotive parts. Galvanizing, predominantly involving zinc coating, is preferred in construction and infrastructure sectors to protect steel from rust and extend the lifespan of structural elements such as beams, pipes, and fencing. Both processes enhance metal durability, but tin-plating excels in fine, precision applications while galvanizing suits heavy-duty, outdoor environments.
Environmental Impact and Sustainability
Tin-plating offers superior corrosion resistance with a lower energy footprint compared to galvanizing, which involves zinc coating and higher greenhouse gas emissions during production. Tin coatings are fully recyclable and non-toxic, reducing environmental hazards often associated with zinc extraction and disposal. Sustainable practices favor tin-plating for applications requiring food safety and prolonged metal preservation due to its minimal ecological impact.
Cost and Longevity Considerations
Tin-plating generally offers a lower initial cost compared to galvanizing, making it suitable for applications requiring moderate corrosion resistance. Galvanizing, using a thicker zinc coating, provides superior longevity by offering robust protection against rust and environmental wear. While tin-plating is quicker and less expensive for small-scale projects, galvanizing ensures a longer lifespan and better durability in harsh outdoor conditions, justifying its higher upfront investment.
Choosing Between Tin-Plating and Galvanizing: Key Factors
Choosing between tin-plating and galvanizing depends on factors such as corrosion resistance, cost-effectiveness, and application environment. Tin-plating offers excellent solderability and is ideal for electronic components, while galvanizing provides superior protection against rust for outdoor and heavy-duty steel structures. Evaluating the specific requirements of durability, conductivity, and aesthetic finish ensures the optimal metal coating is selected.
Tin-Plating vs Galvanizing Infographic
 materialdif.com
materialdif.com