Tin pet solder with Sn (pure tin) offers superior corrosion resistance and a more environmentally friendly choice compared to PbSn (lead-tin) solder. Pure tin provides better wetting capabilities and improved mechanical reliability in electronics assembly, reducing health risks associated with lead exposure. Despite PbSn's lower melting point, modern regulations favor Sn-based solders for safer, long-lasting connections.
Table of Comparison
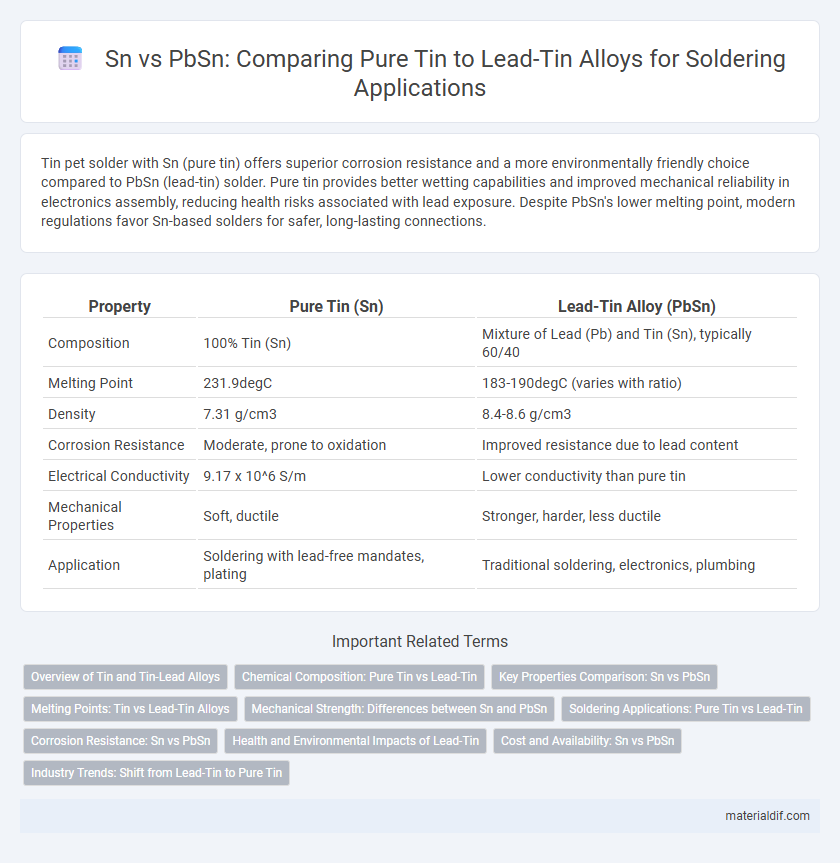
| Property | Pure Tin (Sn) | Lead-Tin Alloy (PbSn) |
|---|---|---|
| Composition | 100% Tin (Sn) | Mixture of Lead (Pb) and Tin (Sn), typically 60/40 |
| Melting Point | 231.9degC | 183-190degC (varies with ratio) |
| Density | 7.31 g/cm3 | 8.4-8.6 g/cm3 |
| Corrosion Resistance | Moderate, prone to oxidation | Improved resistance due to lead content |
| Electrical Conductivity | 9.17 x 10^6 S/m | Lower conductivity than pure tin |
| Mechanical Properties | Soft, ductile | Stronger, harder, less ductile |
| Application | Soldering with lead-free mandates, plating | Traditional soldering, electronics, plumbing |
Overview of Tin and Tin-Lead Alloys
Tin (Sn) is a silvery-white metal known for its corrosion resistance and low toxicity, widely used in soldering and plating applications. Tin-lead (Pb-Sn) alloys, commonly used in traditional solders, combine tin's ductility with lead's low melting point, enhancing mechanical strength and thermal conductivity. While pure tin offers environmental benefits due to its lead-free nature, tin-lead alloys remain prevalent for their superior soldering performance in electronics manufacturing.
Chemical Composition: Pure Tin vs Lead-Tin
Pure tin (Sn) consists of 99.9% tin, offering high corrosion resistance and excellent solderability in electronics. Lead-tin (Pb-Sn) alloys typically combine 60% tin with 40% lead, balancing melting point and mechanical strength for solder applications. The presence of lead enhances thermal fatigue resistance but raises environmental and health concerns due to lead toxicity.
Key Properties Comparison: Sn vs PbSn
Tin (Sn) exhibits higher corrosion resistance and better ductility compared to PbSn (Lead-Tin) alloys, making it ideal for applications requiring durability and malleability. PbSn alloys, typically containing 60% Sn and 40% Pb, offer lower melting points around 183degC, enhancing soldering processes in electronics but sacrificing environmental safety due to lead toxicity. Thermal conductivity is higher in pure tin, promoting efficient heat dissipation, whereas PbSn alloys provide improved mechanical strength but reduced electrical conductivity.
Melting Points: Tin vs Lead-Tin Alloys
Pure tin has a melting point of 231.9degC, while lead-tin alloys exhibit varying melting points depending on their composition. The eutectic alloy of 63% tin and 37% lead melts sharply at 183degC, significantly lower than pure tin, improving soldering efficiency. Understanding these melting point differences is crucial for selecting materials in electronics and metalworking applications.
Mechanical Strength: Differences between Sn and PbSn
PbSn (lead-tin) alloys exhibit higher mechanical strength and better fatigue resistance compared to pure Sn (tin), making them more suitable for applications requiring durability under stress. Pure Sn, while softer and more ductile, is prone to deformation and lower tensile strength. The presence of lead in PbSn alloys significantly enhances hardness and creep resistance, improving overall mechanical performance.
Soldering Applications: Pure Tin vs Lead-Tin
Pure tin offers superior corrosion resistance and reduced toxicity, making it increasingly preferred in modern soldering applications, especially within electronics manufacturing. Lead-tin (PbSn) solder alloys, typically composed of 60% tin and 40% lead, provide lower melting points and better mechanical strength, enhancing joint reliability in traditional circuits. The transition to lead-free solders emphasizes pure tin and its alloys due to stricter environmental regulations such as RoHS, promoting safer and more sustainable soldering solutions.
Corrosion Resistance: Sn vs PbSn
Pure tin (Sn) offers superior corrosion resistance compared to lead-tin (PbSn) alloys, especially in environments exposed to moisture and mild acidic conditions. PbSn alloys often exhibit galvanic corrosion due to the presence of lead, which accelerates material degradation by forming micro-galvanic cells. The enhanced corrosion resistance of pure tin makes it a preferred choice for electronic soldering and protective coating applications where longevity and reliability are critical.
Health and Environmental Impacts of Lead-Tin
Lead-tin (PbSn) alloys pose significant health risks due to lead's toxicity, which can cause neurological damage, kidney problems, and developmental issues in children. Environmentally, lead from PbSn alloys contaminates soil and water, leading to bioaccumulation in wildlife and disrupting ecosystems. In contrast, pure tin (Sn) is non-toxic and environmentally benign, making it a safer alternative for applications requiring food contact or electronics.
Cost and Availability: Sn vs PbSn
Pure tin (Sn) generally costs more than lead-tin (PbSn) alloys due to higher refinement and purity requirements. Lead-tin alloys are more widely available and cheaper because lead is abundant and reduces overall material costs. Cost efficiency and availability of PbSn make it preferred for large-scale soldering applications despite environmental concerns.
Industry Trends: Shift from Lead-Tin to Pure Tin
Industry trends indicate a significant shift from lead-tin (PbSn) solder to pure tin in electronics manufacturing due to stringent environmental regulations like RoHS and the push for lead-free components. Pure tin offers improved compliance with global safety standards and reduces the health hazards associated with lead exposure in industrial settings. Manufacturers are increasingly adopting pure tin to enhance sustainability and meet evolving consumer and regulatory demands.
Sn vs PbSn (Lead-Tin vs Pure Tin) Infographic
 materialdif.com
materialdif.com