Electrolytic tinning involves applying a thin, uniform layer of tin onto metal surfaces using an electric current, resulting in excellent corrosion resistance and a smooth finish suitable for delicate components. Hot-dip tinning, on the other hand, immerses the metal into molten tin, creating a thicker and more robust coating that offers superior protection against wear and harsh environments. Choosing between electrolytic and hot-dip tinning depends on factors like desired coating thickness, application requirements, and cost-efficiency.
Table of Comparison
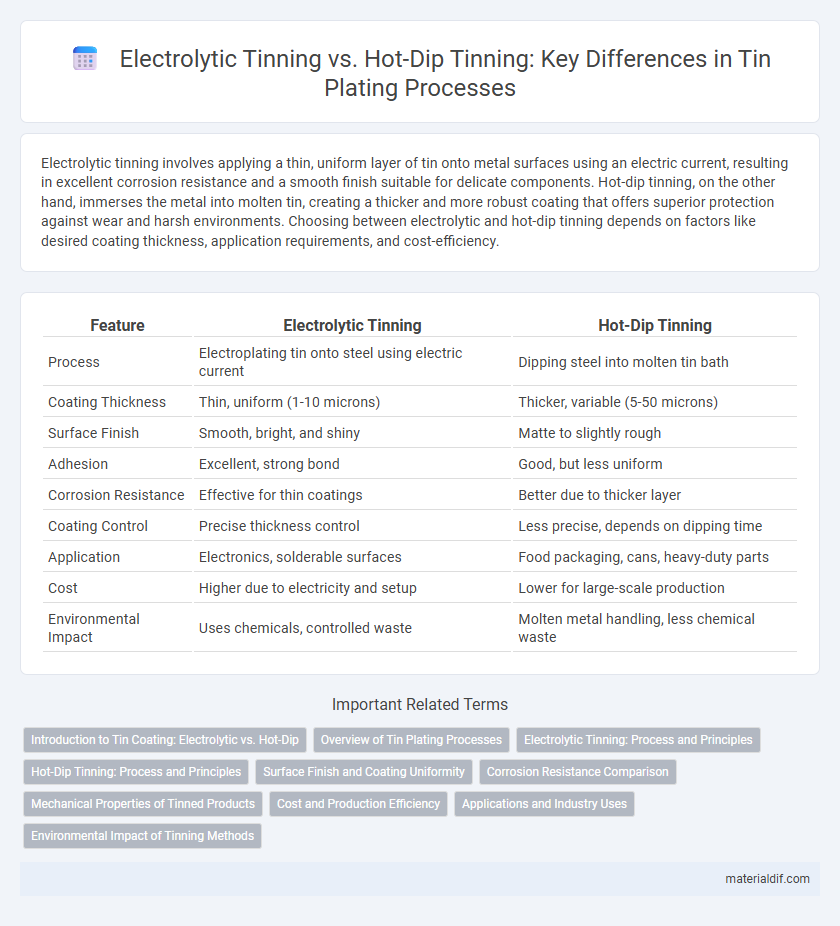
| Feature | Electrolytic Tinning | Hot-Dip Tinning |
|---|---|---|
| Process | Electroplating tin onto steel using electric current | Dipping steel into molten tin bath |
| Coating Thickness | Thin, uniform (1-10 microns) | Thicker, variable (5-50 microns) |
| Surface Finish | Smooth, bright, and shiny | Matte to slightly rough |
| Adhesion | Excellent, strong bond | Good, but less uniform |
| Corrosion Resistance | Effective for thin coatings | Better due to thicker layer |
| Coating Control | Precise thickness control | Less precise, depends on dipping time |
| Application | Electronics, solderable surfaces | Food packaging, cans, heavy-duty parts |
| Cost | Higher due to electricity and setup | Lower for large-scale production |
| Environmental Impact | Uses chemicals, controlled waste | Molten metal handling, less chemical waste |
Introduction to Tin Coating: Electrolytic vs. Hot-Dip
Tin coating techniques include electrolytic tinning, which involves depositing a thin, uniform layer of tin on a metal surface through an electrolytic process, and hot-dip tinning, where the metal is immersed in molten tin to achieve a thicker, more robust coating. Electrolytic tinning is preferred for precision applications requiring fine control over coating thickness, while hot-dip tinning offers superior corrosion resistance and mechanical protection. The choice between these methods depends on factors such as desired coating thickness, application environment, and cost considerations.
Overview of Tin Plating Processes
Electrolytic tinning involves applying a thin, uniform layer of tin onto metal surfaces through electrochemical deposition, providing precise control over coating thickness and superior adhesion. Hot-dip tinning, by immersing metal parts into molten tin at temperatures around 250degC, produces a thicker, robust coating with excellent corrosion resistance and enhanced solderability. Both processes serve critical roles in enhancing metal durability and conductivity, with electrolytic tinning favored for electronic components and hot-dip tinning commonly used in bulk metal protection.
Electrolytic Tinning: Process and Principles
Electrolytic tinning involves depositing a thin, uniform layer of tin onto a metal surface through an electrochemical process, where the metal acts as the cathode in an electrolytic cell containing a tin salt solution. This technique ensures precise control over coating thickness, typically ranging from 1 to 10 micrometers, enhancing corrosion resistance and solderability without the risk of thermal distortion associated with hot-dip tinning. The electrolyte composition, current density, and temperature are critical parameters that influence deposition quality and adhesion in electrolytic tinning.
Hot-Dip Tinning: Process and Principles
Hot-dip tinning involves immersing a metal substrate into molten tin at temperatures typically ranging from 230degC to 260degC, forming a strong metallurgical bond through diffusion between the tin coating and the base metal. This process enhances corrosion resistance and solderability by creating a uniform, adherent tin layer that is thicker and more durable compared to electrolytic tinning. Key principles include precise temperature control and immersion time to ensure optimal coating thickness and minimization of defects like whiskers or uneven deposition.
Surface Finish and Coating Uniformity
Electrolytic tinning produces a smoother and more uniform surface finish due to precise control over deposition parameters, resulting in thinner and more consistent tin coatings ideal for delicate electronic components. Hot-dip tinning often creates a thicker, less uniform layer with slight surface roughness caused by molten tin immersion, suitable for applications requiring robust corrosion resistance. The electrolytic process excels in coating uniformity, minimizing irregularities and enhancing adhesion, whereas hot-dip tinning may exhibit variation in coating thickness due to flux deviation or cooling rates.
Corrosion Resistance Comparison
Electrolytic tinning provides a uniform and thin tin layer that enhances corrosion resistance by minimizing surface defects and ensuring strong adhesion to the base metal. Hot-dip tinning offers a thicker coating, which acts as a robust barrier against corrosive elements but may lead to uneven thickness and potential brittleness over time. In terms of corrosion resistance, electrolytic tinning excels in applications requiring precision and consistent protection, while hot-dip tinning is preferred for heavy-duty environments demanding durable and thicker coatings.
Mechanical Properties of Tinned Products
Electrolytic tinning produces a thin, uniform tin layer that enhances surface hardness and provides superior adhesion, resulting in improved wear resistance and durability. Hot-dip tinning creates a thicker coating with a more robust metallurgical bond, offering excellent impact resistance and fatigue strength in tinned products. Both methods influence mechanical properties differently, with electrolytic tinning favoring precision applications and hot-dip tinning suited for heavy-duty protection.
Cost and Production Efficiency
Electrolytic tinning offers higher production efficiency with precise control over tin thickness and reduced material waste, making it cost-effective for small to medium batch sizes. Hot-dip tinning, while generally lower in initial equipment cost, involves longer processing times and higher energy consumption, which can increase operational expenses for large-scale production. Choosing between these methods depends on balancing upfront investment with ongoing costs and production volume requirements.
Applications and Industry Uses
Electrolytic tinning provides precise and uniform tin coatings ideal for electronic components, ensuring superior conductivity and corrosion resistance in the semiconductor and circuit board industries. Hot-dip tinning offers thicker, more durable layers suitable for robust applications like food packaging, automotive parts, and steel wire protection, where mechanical strength and long-term corrosion resistance are critical. Both methods support diverse industrial needs by enhancing metal surfaces, with electrolytic tinning favored in high-tech manufacturing and hot-dip tinning in heavy-duty metal preservation.
Environmental Impact of Tinning Methods
Electrolytic tinning produces less waste and lower energy consumption compared to hot-dip tinning, resulting in reduced environmental pollution. Hot-dip tinning generates hazardous sludge and emits higher levels of volatile organic compounds (VOCs) and heavy metals, posing greater risks to ecosystems. Recycling opportunities and efficient wastewater treatment in electrolytic systems further minimize environmental footprint.
Electrolytic tinning vs hot-dip tinning Infographic
 materialdif.com
materialdif.com