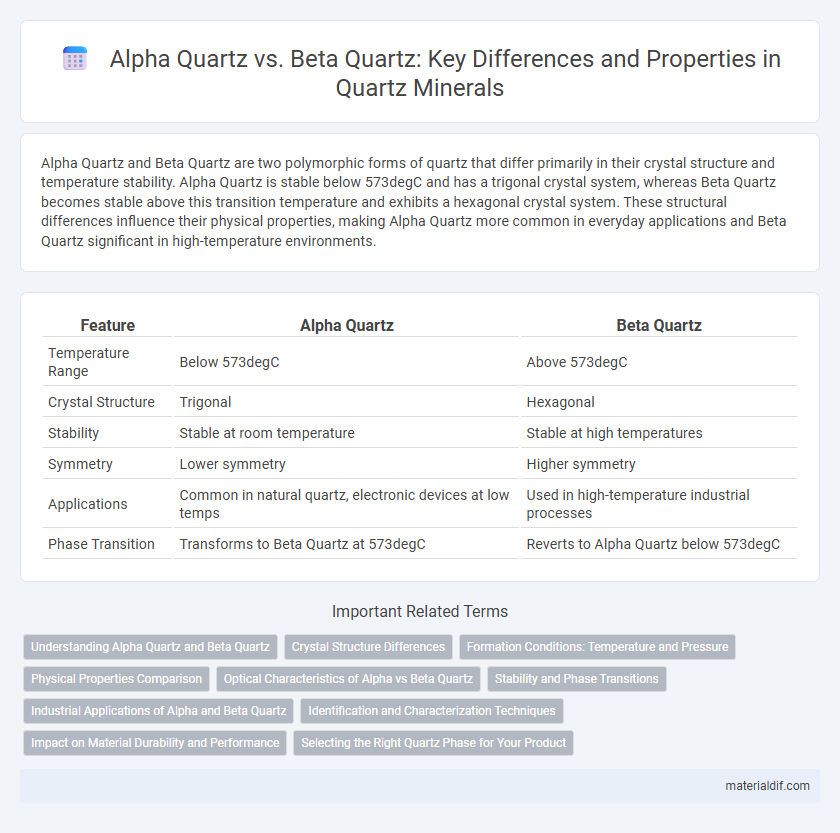
Alpha Quartz and Beta Quartz are two polymorphic forms of quartz that differ primarily in their crystal structure and temperature stability. Alpha Quartz is stable below 573degC and has a trigonal crystal system, whereas Beta Quartz becomes stable above this transition temperature and exhibits a hexagonal crystal system. These structural differences influence their physical properties, making Alpha Quartz more common in everyday applications and Beta Quartz significant in high-temperature environments.
Table of Comparison
| Feature | Alpha Quartz | Beta Quartz |
|---|---|---|
| Temperature Range | Below 573degC | Above 573degC |
| Crystal Structure | Trigonal | Hexagonal |
| Stability | Stable at room temperature | Stable at high temperatures |
| Symmetry | Lower symmetry | Higher symmetry |
| Applications | Common in natural quartz, electronic devices at low temps | Used in high-temperature industrial processes |
| Phase Transition | Transforms to Beta Quartz at 573degC | Reverts to Alpha Quartz below 573degC |
Understanding Alpha Quartz and Beta Quartz
Alpha quartz and beta quartz are two distinct polymorphs of silicon dioxide (SiO2) characterized by different crystal structures and temperature stability ranges. Alpha quartz is stable below 573degC with a trigonal crystal system, exhibiting piezoelectric properties widely used in electronic applications. Beta quartz forms above 573degC, possessing a hexagonal structure and greater symmetry, transitioning back to alpha quartz upon cooling, which is essential for geological and industrial processes involving heat.
Crystal Structure Differences
Alpha quartz exhibits a trigonal crystal structure characterized by a stable, low-temperature form, while beta quartz transforms into a hexagonal structure at temperatures above 573degC. The transition between alpha and beta quartz involves a reversible change in lattice parameters, affecting the symmetry and volume of the crystal. This structural difference influences physical properties such as thermal expansion and piezoelectricity, critical in various industrial applications.
Formation Conditions: Temperature and Pressure
Alpha quartz forms at lower temperatures below 573degC and under relatively low pressure conditions, stabilizing its trigonal crystal structure. Beta quartz appears when temperatures exceed 573degC, maintaining its hexagonal crystal system under similar low-pressure environments. The reversible phase transformation between alpha and beta quartz is driven primarily by changes in temperature rather than pressure fluctuations.
Physical Properties Comparison
Alpha quartz exhibits a trigonal crystal system with lower symmetry and is stable at temperatures below 573degC, while beta quartz forms a hexagonal crystal system with higher symmetry and is stable above this transition temperature. The specific gravity of alpha quartz is approximately 2.65, slightly differing in beta quartz due to thermal expansion. Both forms share similar hardness around 7 on the Mohs scale, but beta quartz displays increased thermal stability and elasticity at elevated temperatures.
Optical Characteristics of Alpha vs Beta Quartz
Alpha quartz exhibits uniaxial positive optical properties with a refractive index range of approximately 1.544 to 1.553, while beta quartz, stable above 573degC, shows a uniaxial negative optic sign with slightly lower refractive indices around 1.54. The transition from alpha to beta quartz involves a shift in crystal symmetry from trigonal to hexagonal, causing changes in birefringence and optical behavior under polarized light. These distinct optical characteristics enable precise identification and differentiation of alpha and beta quartz phases in petrographic analysis.
Stability and Phase Transitions
Alpha quartz is the stable form of quartz at temperatures below 573degC, characterized by a trigonal crystal structure, while beta quartz is stable above this temperature and exhibits a hexagonal structure. The phase transition from alpha to beta quartz at 573degC is reversible and involves a rapid rearrangement of the silica tetrahedra, affecting the material's thermal expansion and optical properties. Understanding this stability and phase transition behavior is critical for applications in electronics, optics, and geoscience.
Industrial Applications of Alpha and Beta Quartz
Alpha quartz, stable below 573degC, is widely used in precision instruments, oscillators, and sensors due to its piezoelectric properties, ensuring dimensional stability at typical operating temperatures. Beta quartz, stable above 573degC, finds specialized applications in high-temperature ceramics and refractory materials but is less common in electronics owing to its higher temperature phase. Industrial reliance on alpha quartz dominates because of its reliable performance in electronic components and timekeeping devices.
Identification and Characterization Techniques
Alpha quartz and beta quartz differ primarily in crystalline structure, identifiable using X-ray diffraction (XRD) which reveals alpha quartz's trigonal symmetry versus beta quartz's hexagonal symmetry. Raman spectroscopy further characterizes these phases by detecting variations in vibrational modes specific to each structure, with alpha quartz showing sharper peaks in the 200-500 cm-1 range compared to beta quartz. Differential scanning calorimetry (DSC) complements these techniques by detecting the reversible phase transition near 573degC, confirming identification through thermal behavior differences.
Impact on Material Durability and Performance
Alpha quartz, stable at temperatures below 573degC, exhibits excellent hardness and structural integrity, enhancing material durability in applications such as electronic components and glass manufacturing. Beta quartz transforms into a less stable hexagonal crystal structure above 573degC, which can reduce mechanical strength and lead to performance degradation under high thermal stress. The reversible phase transition between alpha and beta quartz impacts thermal expansion and stress resistance, critical factors in the longevity and reliability of quartz-based materials.
Selecting the Right Quartz Phase for Your Product
Alpha quartz is stable at temperatures below 573degC and is ideal for applications requiring low thermal expansion and high mechanical strength. Beta quartz forms above 573degC and is favored in high-temperature environments due to its increased thermal expansion and altered crystal symmetry. Selecting the right quartz phase depends on the operational temperature range and mechanical demands of your product to ensure optimal performance and durability.
Alpha Quartz vs Beta Quartz Infographic
 materialdif.com
materialdif.com