Chain scission in polymers refers to the breaking of polymer chains, leading to a reduction in molecular weight and deterioration of mechanical properties. Cross-linking involves the formation of covalent bonds between polymer chains, enhancing rigidity, thermal stability, and resistance to solvents. Understanding the balance between chain scission and cross-linking is crucial for controlling the physical properties and performance of polymer materials in various applications.
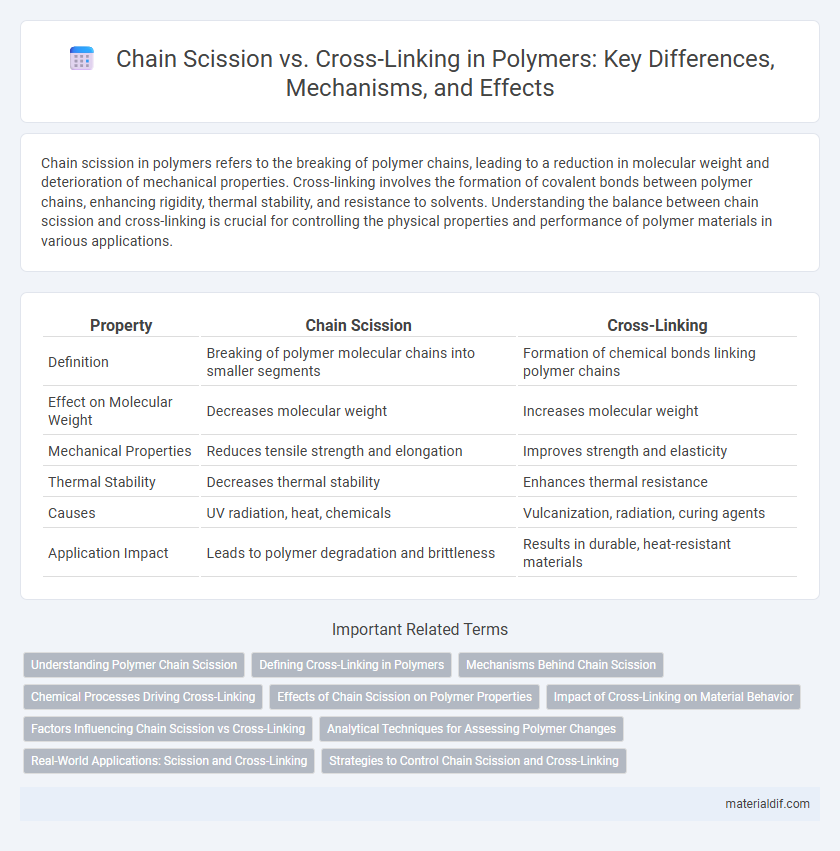
Table of Comparison
| Property | Chain Scission | Cross-Linking |
|---|---|---|
| Definition | Breaking of polymer molecular chains into smaller segments | Formation of chemical bonds linking polymer chains |
| Effect on Molecular Weight | Decreases molecular weight | Increases molecular weight |
| Mechanical Properties | Reduces tensile strength and elongation | Improves strength and elasticity |
| Thermal Stability | Decreases thermal stability | Enhances thermal resistance |
| Causes | UV radiation, heat, chemicals | Vulcanization, radiation, curing agents |
| Application Impact | Leads to polymer degradation and brittleness | Results in durable, heat-resistant materials |
Understanding Polymer Chain Scission
Polymer chain scission refers to the breaking of covalent bonds within the polymer backbone, leading to a reduction in molecular weight and mechanical strength. This degradation process occurs due to thermal, chemical, or mechanical stress, resulting in shorter polymer chains and altered physical properties. Monitoring chain scission is critical for predicting polymer lifespan and performance in applications such as plastics, elastomers, and composites.
Defining Cross-Linking in Polymers
Cross-linking in polymers refers to the formation of covalent bonds between polymer chains, creating a three-dimensional network structure. This process enhances mechanical strength, thermal resistance, and chemical stability by restricting chain mobility and increasing molecular weight. Cross-linking can be induced through chemical agents, radiation, or heat, significantly influencing the polymer's physical properties and performance.
Mechanisms Behind Chain Scission
Chain scission in polymers occurs through the breaking of covalent bonds within the polymer backbone, typically induced by thermal, mechanical, or chemical stress. Free radical formation is a primary mechanism, where radicals attack polymer chains causing bond cleavage and resulting in reduced molecular weight. This degradation process contrasts with cross-linking, which forms covalent bonds between chains, increasing polymer network density and mechanical strength.
Chemical Processes Driving Cross-Linking
Chemical processes driving cross-linking in polymers involve the formation of covalent bonds between polymer chains, enhancing molecular weight and network density. Reactive species such as free radicals, ions, or catalysts induce these linkages by initiating polymer chain interactions, leading to three-dimensional network structures that improve mechanical strength and thermal stability. Cross-linking contrasts with chain scission, which reduces molecular weight by breaking polymer chains, impacting properties like tensile strength and elasticity.
Effects of Chain Scission on Polymer Properties
Chain scission in polymers results in the cleavage of molecular chains, significantly reducing molecular weight and leading to decreased mechanical strength and toughness. This degradation process increases polymer brittleness and diminishes elongation at break, impairing flexibility and durability. The reduction in chain length also affects thermal stability and chemical resistance, accelerating polymer aging and failure in applications.
Impact of Cross-Linking on Material Behavior
Cross-linking in polymers significantly enhances mechanical strength, thermal stability, and chemical resistance by creating covalent bonds between polymer chains, forming a three-dimensional network. This interconnected network reduces polymer chain mobility, resulting in increased rigidity and reduced solubility. Cross-linked polymers exhibit improved durability and are less prone to degradation compared to materials undergoing chain scission, which typically leads to decreased molecular weight and mechanical properties.
Factors Influencing Chain Scission vs Cross-Linking
Factors influencing chain scission versus cross-linking in polymers include temperature, radiation type and intensity, and polymer chemical structure. High-energy radiation and elevated temperatures typically promote chain scission by breaking covalent bonds, while certain catalysts and reactive environments favor cross-linking through bond formation between polymer chains. Molecular weight, presence of oxygen, and polymer morphology also critically affect the balance between degradation and network formation mechanisms.
Analytical Techniques for Assessing Polymer Changes
Chain scission and cross-linking in polymers can be accurately assessed using techniques such as gel permeation chromatography (GPC), which measures molecular weight distributions to detect chain breakage or network formation. Fourier transform infrared spectroscopy (FTIR) identifies chemical bond changes indicative of cross-linking or degradation. Rheological analysis further distinguishes alterations in polymer viscosity and elasticity, revealing modifications in molecular architecture.
Real-World Applications: Scission and Cross-Linking
Chain scission in polymers breaks molecular chains, leading to reduced molecular weight and decreased mechanical strength, which is critical in applications like recycling thermoplastics where controlled degradation is desired. Cross-linking forms covalent bonds between polymer chains, enhancing thermal stability, elasticity, and chemical resistance, essential for producing vulcanized rubber and epoxy resins used in automotive and aerospace industries. Understanding the balance between chain scission and cross-linking enables the design of polymers with tailored properties for specific real-world applications such as durable coatings and high-performance composites.
Strategies to Control Chain Scission and Cross-Linking
Strategies to control chain scission and cross-linking in polymers include optimizing reaction conditions such as temperature and radiation dose to minimize unwanted molecular degradation. Incorporating stabilizers like antioxidants and UV absorbers effectively inhibits chain scission by neutralizing free radicals. Tailoring cross-linking agents and catalysts ensures precise network formation, enhancing mechanical properties while preventing excessive cross-link density that could cause brittleness.
Chain Scission vs Cross-Linking Infographic
 materialdif.com
materialdif.com