Addition polymerization involves the repetitive linking of monomers with double bonds, producing polymers without the loss of any small molecules. Condensation polymerization forms polymers through a reaction between monomers with two functional groups, releasing small byproducts such as water or methanol. The main difference lies in the mechanism and presence of byproducts, impacting the properties and applications of the resulting polymers.
Table of Comparison
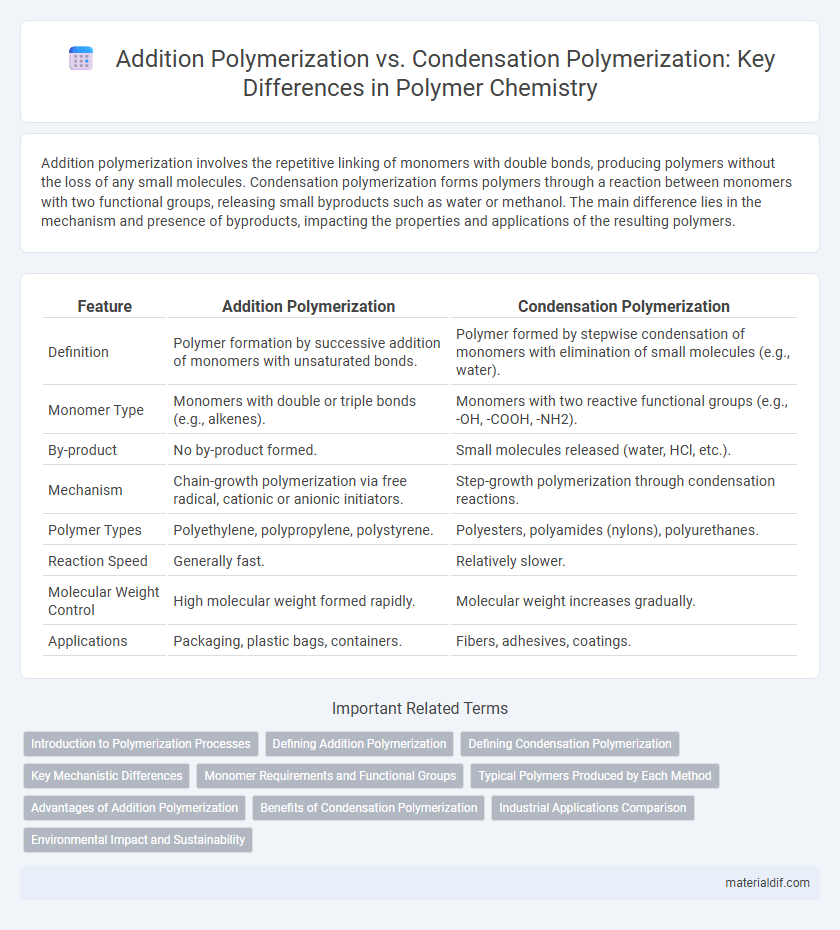
| Feature | Addition Polymerization | Condensation Polymerization |
|---|---|---|
| Definition | Polymer formation by successive addition of monomers with unsaturated bonds. | Polymer formed by stepwise condensation of monomers with elimination of small molecules (e.g., water). |
| Monomer Type | Monomers with double or triple bonds (e.g., alkenes). | Monomers with two reactive functional groups (e.g., -OH, -COOH, -NH2). |
| By-product | No by-product formed. | Small molecules released (water, HCl, etc.). |
| Mechanism | Chain-growth polymerization via free radical, cationic or anionic initiators. | Step-growth polymerization through condensation reactions. |
| Polymer Types | Polyethylene, polypropylene, polystyrene. | Polyesters, polyamides (nylons), polyurethanes. |
| Reaction Speed | Generally fast. | Relatively slower. |
| Molecular Weight Control | High molecular weight formed rapidly. | Molecular weight increases gradually. |
| Applications | Packaging, plastic bags, containers. | Fibers, adhesives, coatings. |
Introduction to Polymerization Processes
Addition polymerization involves the successive linking of monomers with unsaturated bonds such as alkenes, forming polymers without the loss of small molecules. Condensation polymerization occurs when monomers with two or more reactive end groups combine, releasing small molecules like water or methanol during polymer chain formation. These fundamental polymerization processes dictate the structural properties and applications of polymers across industries.
Defining Addition Polymerization
Addition polymerization is a process where monomers with unsaturated bonds, typically alkenes, react to form polymers by successive addition without the loss of any small molecules. This mechanism involves the breaking of double bonds and the formation of a long chain through free radicals, cations, or anions as initiators. Unlike condensation polymerization, addition polymerization retains the full molecular weight of the monomers in the polymer structure, resulting in high molecular weight polymers such as polyethylene and polystyrene.
Defining Condensation Polymerization
Condensation polymerization is a chemical process where monomers join together through the elimination of small molecules such as water or methanol, forming polymers with repeating units linked by covalent bonds. This method contrasts with addition polymerization by involving step-growth mechanisms and often producing polymers like polyesters, polyamides, and polyurethanes. The characteristic feature of condensation polymerization is the production of by-products, making it essential in synthesizing biopolymers and high-performance materials.
Key Mechanistic Differences
Addition polymerization involves the successive addition of monomer units with double bonds, typically initiated by free radicals, resulting in polymers without by-products. Condensation polymerization proceeds through a step-growth mechanism where monomers with functional groups react to form polymers and small molecules like water or methanol are released as by-products. The key mechanistic difference lies in addition polymerization's chain-growth process versus condensation polymerization's step-growth process involving elimination reactions.
Monomer Requirements and Functional Groups
Addition polymerization requires monomers with double bonds, such as alkenes, which open up to form long chains without losing atoms, preserving the original monomer units. Condensation polymerization involves monomers with two different functional groups, like diols and dicarboxylic acids, that react to form polymers with the elimination of small molecules such as water. The key difference lies in the monomer functionality: addition polymerization uses unsaturated monomers with reactive double bonds, while condensation polymerization relies on bifunctional or polyfunctional monomers capable of forming covalent bonds through functional group interactions.
Typical Polymers Produced by Each Method
Addition polymerization typically produces polymers such as polyethylene, polypropylene, polystyrene, and polyvinyl chloride (PVC), characterized by monomers with double bonds that open up and link without by-products. Condensation polymerization yields polymers like nylon, polyester, and polyurethane, formed through step-growth reactions releasing small molecules such as water or methanol as by-products. These distinctions influence polymer properties and applications, with addition polymers often used in packaging and insulation, while condensation polymers find roles in textiles and automotive components.
Advantages of Addition Polymerization
Addition polymerization offers notable advantages, such as rapid reaction rates and the ability to produce high-molecular-weight polymers without generating byproducts. This process ensures greater control over polymer structure and molecular weight distribution, leading to consistent material properties. Common examples include polyethylene and polypropylene, widely utilized for their durability and versatility in various industrial applications.
Benefits of Condensation Polymerization
Condensation polymerization offers the advantage of producing polymers with well-defined molecular weights and functional end groups, enabling precise control over polymer architecture and properties. This method efficiently generates water or small molecule by-products, facilitating purification and enabling sustainable processing. Polymers formed via condensation polymerization often exhibit enhanced thermal stability and mechanical strength, making them ideal for high-performance applications such as engineering plastics and biodegradable materials.
Industrial Applications Comparison
Addition polymerization dominates industries producing polyethylene and polypropylene due to its rapid reaction rates and high molecular weight polymers suitable for packaging and automotive parts. Condensation polymerization is essential in manufacturing polyesters and polyamides, offering superior thermal stability and mechanical strength critical for textiles and engineering plastics. Industrial selection depends on material properties, processing conditions, and end-use applications, with addition polymers favored for large-scale commodity production and condensation polymers preferred for specialty, high-performance materials.
Environmental Impact and Sustainability
Addition polymerization generally produces fewer byproducts and typically involves monomers like ethylene or propylene, which can be sourced from non-renewable fossil fuels, resulting in polymers such as polyethylene and polypropylene with relatively lower environmental degradation during manufacturing. Condensation polymerization often generates small molecule byproducts, such as water or methanol, and can utilize renewable resources like diols and dicarboxylic acids, exemplified by polyesters and polyamides, but the process may involve higher energy consumption and challenges in biodegradability. Sustainable polymer development increasingly focuses on bio-based monomers and recyclable designs, aiming to reduce carbon footprint and environmental persistence regardless of polymerization route.
Addition Polymerization vs Condensation Polymerization Infographic
 materialdif.com
materialdif.com