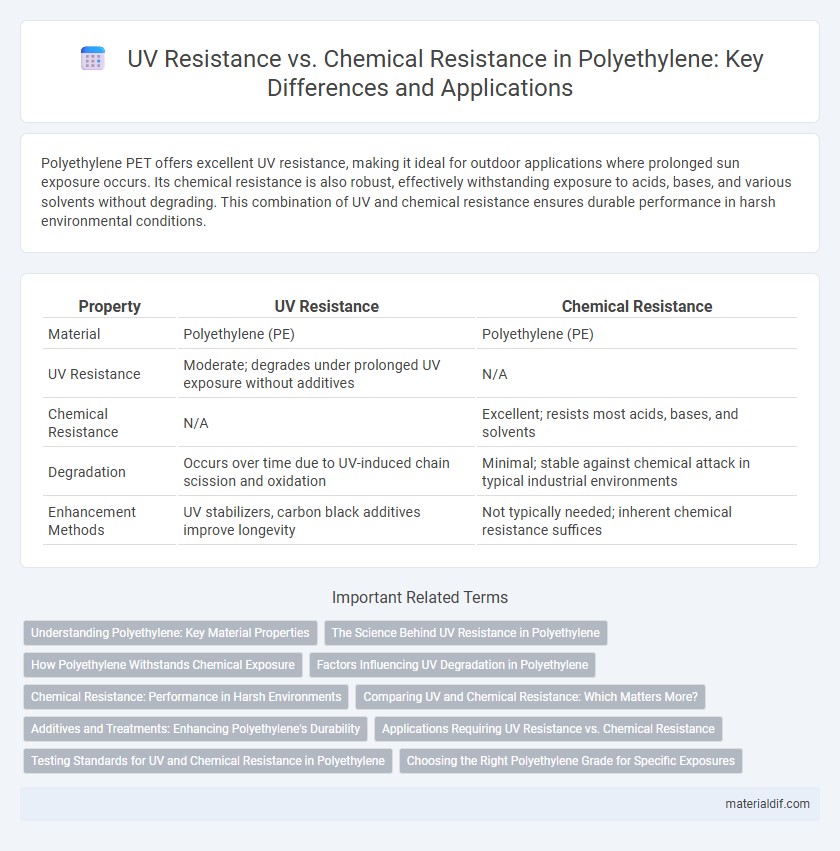
Polyethylene PET offers excellent UV resistance, making it ideal for outdoor applications where prolonged sun exposure occurs. Its chemical resistance is also robust, effectively withstanding exposure to acids, bases, and various solvents without degrading. This combination of UV and chemical resistance ensures durable performance in harsh environmental conditions.
Table of Comparison
| Property | UV Resistance | Chemical Resistance |
|---|---|---|
| Material | Polyethylene (PE) | Polyethylene (PE) |
| UV Resistance | Moderate; degrades under prolonged UV exposure without additives | N/A |
| Chemical Resistance | N/A | Excellent; resists most acids, bases, and solvents |
| Degradation | Occurs over time due to UV-induced chain scission and oxidation | Minimal; stable against chemical attack in typical industrial environments |
| Enhancement Methods | UV stabilizers, carbon black additives improve longevity | Not typically needed; inherent chemical resistance suffices |
Understanding Polyethylene: Key Material Properties
Polyethylene exhibits excellent chemical resistance, effectively resisting acids, bases, and solvents, which makes it ideal for various industrial applications involving aggressive chemicals. Its UV resistance, while generally good in high-density polyethylene (HDPE), varies and often requires additives or stabilizers to prevent degradation under prolonged sunlight exposure. Understanding these distinctions helps in selecting the right grade of polyethylene for specific environments, balancing durability against chemical attack and UV exposure.
The Science Behind UV Resistance in Polyethylene
Polyethylene's UV resistance stems from its molecular structure, where the saturated carbon-carbon bonds absorb and dissipate UV radiation, minimizing photo-degradation. Stabilizers such as carbon black and hindered amine light stabilizers (HALS) enhance this resistance by scavenging free radicals generated during UV exposure. In contrast, chemical resistance in polyethylene is primarily due to its non-polar, hydrophobic nature, which prevents interaction with polar solvents and chemicals, making it effective against acids, bases, and organic solvents.
How Polyethylene Withstands Chemical Exposure
Polyethylene exhibits exceptional chemical resistance due to its non-polar molecular structure, allowing it to withstand exposure to acids, bases, and solvents without significant degradation. Its resistance to UV radiation is moderate, requiring additives or stabilizers to enhance performance under prolonged sunlight exposure. This inherent chemical stability makes polyethylene a preferred material for containers and piping in harsh chemical environments.
Factors Influencing UV Degradation in Polyethylene
UV degradation in polyethylene is primarily influenced by the polymer's molecular structure, presence of additives such as UV stabilizers, and environmental exposure factors including sunlight intensity and oxygen availability. Chemical resistance varies with polyethylene's density and crystallinity, but UV resistance mainly depends on the efficiency of hindered amine light stabilizers (HALS) and carbon black content. Prolonged UV exposure generates free radicals that break polymer chains, accelerated by high temperatures and moisture, leading to discoloration and mechanical property deterioration.
Chemical Resistance: Performance in Harsh Environments
Polyethylene exhibits exceptional chemical resistance, making it highly effective in harsh environments containing acids, bases, and solvents. Its molecular structure prevents degradation and elongates the lifespan of products exposed to corrosive chemicals. This chemical stability outperforms many other polymers, ensuring reliability in industrial and marine applications.
Comparing UV and Chemical Resistance: Which Matters More?
Polyethylene exhibits strong chemical resistance, making it highly effective against acids, bases, and solvents, which ensures durability in harsh chemical environments. However, its natural UV resistance is limited, often requiring additives or coatings to prevent degradation and maintain mechanical properties under prolonged sunlight exposure. Choosing between UV and chemical resistance depends on application conditions, but chemical resistance generally plays a more critical role in preserving polyethylene's structural integrity in industrial uses.
Additives and Treatments: Enhancing Polyethylene's Durability
Additives such as UV stabilizers and hindered amine light stabilizers (HALS) significantly improve polyethylene's resistance to ultraviolet degradation, extending its lifespan under sunlight exposure. Chemical resistance in polyethylene is enhanced through specific fillers and antioxidants that protect the polymer matrix from harsh solvents and oxidizing agents. Treatments like cross-linking and surface coatings further bolster durability by creating barriers against environmental stressors, optimizing polyethylene's performance in demanding applications.
Applications Requiring UV Resistance vs. Chemical Resistance
Polyethylene demonstrates superior UV resistance in applications exposed to prolonged sunlight, such as outdoor geomembranes, agricultural films, and automotive components, where degradation from UV light can compromise material integrity. In contrast, chemical resistance makes it ideal for use in piping systems, chemical storage tanks, and industrial containers that handle corrosive substances and solvents. Selecting polyethylene grades with enhanced UV stabilizers or chemical resistance additives is crucial to tailoring performance for specific environmental conditions and operational demands.
Testing Standards for UV and Chemical Resistance in Polyethylene
Testing standards for UV resistance in polyethylene typically include ASTM G154 and ISO 4892, which simulate accelerated weathering through controlled exposure to UV light and moisture. Chemical resistance assessments often follow ASTM D543, focusing on the material's performance when immersed in various chemicals to evaluate degradation or swelling. These standardized tests provide critical data for selecting polyethylene grades suitable for outdoor applications requiring durable UV protection alongside chemical exposure resilience.
Choosing the Right Polyethylene Grade for Specific Exposures
Selecting the appropriate polyethylene grade depends on the intended exposure environment, as UV-resistant grades incorporate additives like carbon black to enhance durability against sunlight degradation. Chemical resistance varies among low-density polyethylene (LDPE) and high-density polyethylene (HDPE), with HDPE exhibiting superior resistance to acids, bases, and organic solvents. For applications involving prolonged outdoor use combined with chemical exposure, HDPE with UV stabilizers offers optimal performance and longevity.
UV resistance vs Chemical resistance Infographic
 materialdif.com
materialdif.com