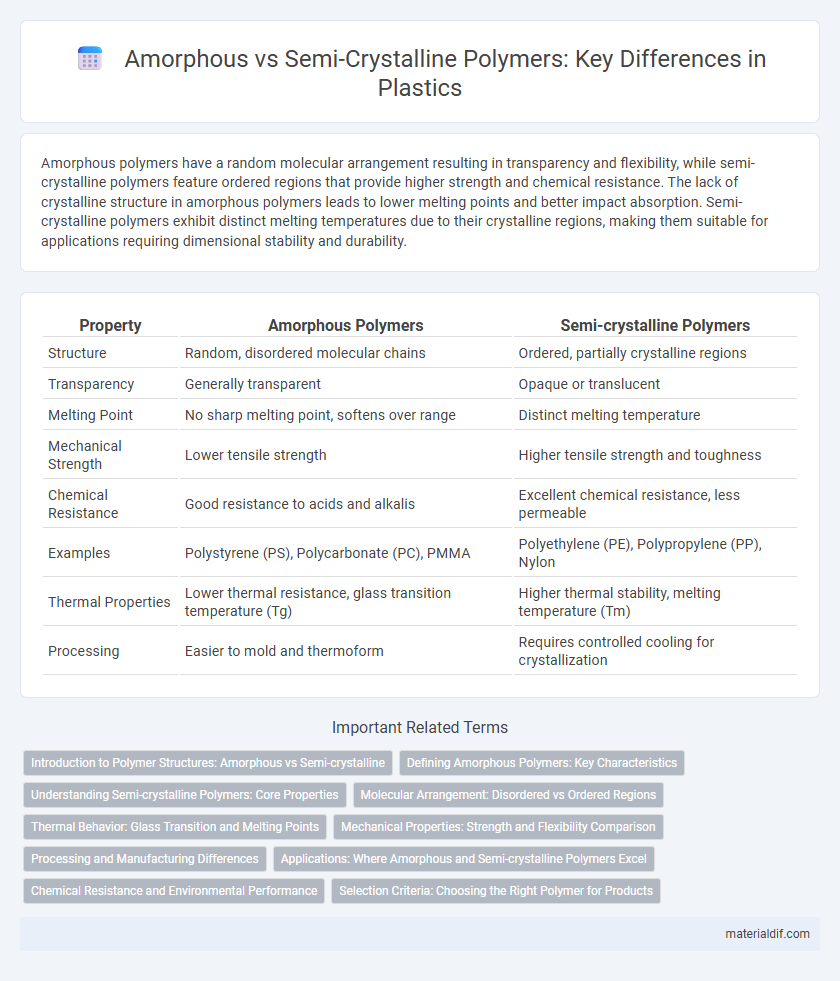
Amorphous polymers have a random molecular arrangement resulting in transparency and flexibility, while semi-crystalline polymers feature ordered regions that provide higher strength and chemical resistance. The lack of crystalline structure in amorphous polymers leads to lower melting points and better impact absorption. Semi-crystalline polymers exhibit distinct melting temperatures due to their crystalline regions, making them suitable for applications requiring dimensional stability and durability.
Table of Comparison
| Property | Amorphous Polymers | Semi-crystalline Polymers |
|---|---|---|
| Structure | Random, disordered molecular chains | Ordered, partially crystalline regions |
| Transparency | Generally transparent | Opaque or translucent |
| Melting Point | No sharp melting point, softens over range | Distinct melting temperature |
| Mechanical Strength | Lower tensile strength | Higher tensile strength and toughness |
| Chemical Resistance | Good resistance to acids and alkalis | Excellent chemical resistance, less permeable |
| Examples | Polystyrene (PS), Polycarbonate (PC), PMMA | Polyethylene (PE), Polypropylene (PP), Nylon |
| Thermal Properties | Lower thermal resistance, glass transition temperature (Tg) | Higher thermal stability, melting temperature (Tm) |
| Processing | Easier to mold and thermoform | Requires controlled cooling for crystallization |
Introduction to Polymer Structures: Amorphous vs Semi-crystalline
Amorphous polymers exhibit a random, disordered molecular arrangement, resulting in transparent and flexible materials with lower density and melting points. Semi-crystalline polymers feature highly ordered, tightly packed crystalline regions interspersed with amorphous zones, providing enhanced mechanical strength and chemical resistance. Common examples include polystyrene for amorphous and polyethylene for semi-crystalline polymers, highlighting their distinct thermal and physical properties in applications.
Defining Amorphous Polymers: Key Characteristics
Amorphous polymers are characterized by a random, disordered molecular structure lacking a defined crystalline arrangement, resulting in transparent and flexible materials with lower density. Their irregular chain packing leads to isotropic physical properties and a broad glass transition temperature rather than a sharp melting point. Common examples include polystyrene (PS), polycarbonate (PC), and polymethyl methacrylate (PMMA), widely used in applications requiring optical clarity and impact resistance.
Understanding Semi-crystalline Polymers: Core Properties
Semi-crystalline polymers exhibit distinct regions of ordered crystalline structures interspersed with amorphous areas, resulting in enhanced mechanical strength, thermal resistance, and chemical stability compared to purely amorphous polymers. Key examples include polyethylene, polypropylene, and polyoxymethylene, widely used in industries requiring durability and dimensional stability. Their melting temperature, degree of crystallinity, and spherulite morphology critically influence product performance in applications such as packaging, automotive components, and medical devices.
Molecular Arrangement: Disordered vs Ordered Regions
Amorphous polymers exhibit a molecular arrangement characterized by disordered, random chains that lack a defined structure, resulting in isotropic properties and increased transparency. In contrast, semi-crystalline polymers contain both ordered crystalline regions and disordered amorphous areas, imparting higher density, strength, and thermal resistance due to the regular packing of molecular chains. The degree of crystallinity directly influences mechanical performance and chemical resistance, making semi-crystalline polymers preferred in applications requiring durability and dimensional stability.
Thermal Behavior: Glass Transition and Melting Points
Amorphous polymers exhibit a distinct glass transition temperature (Tg) where they transition from rigid to rubbery states without a clear melting point, reflecting their disordered molecular structure. Semi-crystalline polymers possess both a glass transition temperature and a sharp melting point (Tm) due to their ordered crystalline regions, which require higher energy to disrupt. The thermal behavior differences impact processing temperatures and thermal stability, with semi-crystalline polymers generally demonstrating superior heat resistance and dimensional stability compared to amorphous polymers.
Mechanical Properties: Strength and Flexibility Comparison
Amorphous polymers exhibit lower tensile strength but higher flexibility due to their random molecular arrangement, allowing for greater impact resistance and deformation without fracture. Semi-crystalline polymers possess higher tensile strength and stiffness because of their ordered, tightly packed crystalline regions, which enhance load-bearing capacity but reduce flexibility. The balance between crystallinity and amorphous regions significantly influences a polymer's mechanical properties, affecting applications requiring either rigidity or elasticity.
Processing and Manufacturing Differences
Amorphous polymers, such as polystyrene and polycarbonate, exhibit random molecular chains that provide excellent transparency and easier melt processing at lower temperatures, making them ideal for injection molding and extrusion with precise dimensional control. Semi-crystalline polymers, including polyethylene and polypropylene, have ordered molecular regions that confer higher mechanical strength and chemical resistance but require slower cooling rates and higher processing temperatures to achieve optimal crystallinity and reduce warpage. The manufacturing of semi-crystalline polymers often involves post-processing annealing to enhance structural stability, whereas amorphous polymers benefit from faster cycle times due to their uniform cooling behavior.
Applications: Where Amorphous and Semi-crystalline Polymers Excel
Amorphous polymers excel in applications requiring transparency, impact resistance, and ease of processing, making them suitable for optical lenses, packaging films, and medical devices. Semi-crystalline polymers offer superior chemical resistance, mechanical strength, and thermal stability, which are critical for automotive parts, piping systems, and industrial containers. Selection depends on balancing clarity and flexibility versus durability and heat resistance in targeted applications.
Chemical Resistance and Environmental Performance
Amorphous polymers exhibit superior chemical resistance due to their random molecular structure, which limits solvent penetration and reduces swelling. Semi-crystalline polymers offer enhanced environmental performance with higher mechanical strength and thermal stability, owing to their ordered crystalline regions that resist degradation. Both polymer types demonstrate distinct advantages, with amorphous polymers favored in applications requiring chemical inertness and semi-crystalline polymers preferred for durability in challenging environmental conditions.
Selection Criteria: Choosing the Right Polymer for Products
Amorphous polymers offer transparency, flexibility, and impact resistance, making them ideal for applications requiring optical clarity and toughness, such as lenses and flexible packaging. Semi-crystalline polymers provide higher chemical resistance, mechanical strength, and thermal stability, suited for structural parts and high-performance components like automotive parts and medical devices. Selection criteria prioritize the balance between mechanical properties, thermal performance, and environmental exposure to optimize product durability and functionality.
Amorphous Polymers vs Semi-crystalline Polymers Infographic
 materialdif.com
materialdif.com