Nickel sulfide and nickel oxide differ significantly in their chemical properties and industrial applications. Nickel sulfide is commonly found in ore deposits and is primarily used as a precursor in nickel extraction, while nickel oxide serves as a catalyst and is essential in battery manufacturing. Understanding these differences is crucial for optimizing processes in metallurgy and energy storage technologies.
Table of Comparison
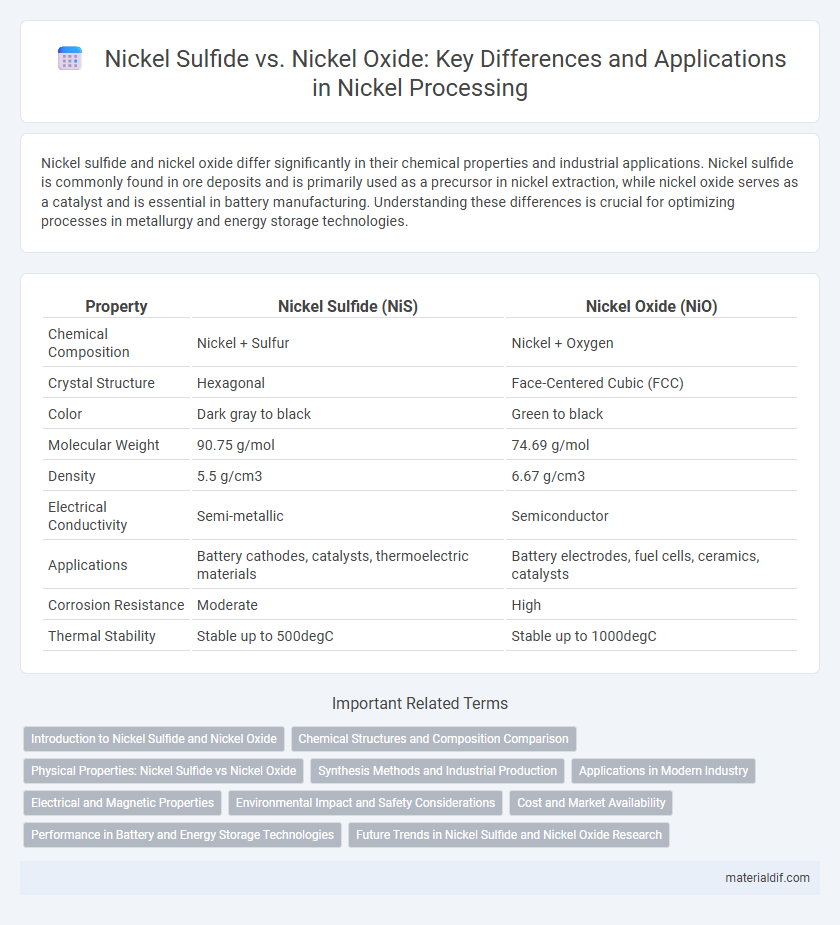
| Property | Nickel Sulfide (NiS) | Nickel Oxide (NiO) |
|---|---|---|
| Chemical Composition | Nickel + Sulfur | Nickel + Oxygen |
| Crystal Structure | Hexagonal | Face-Centered Cubic (FCC) |
| Color | Dark gray to black | Green to black |
| Molecular Weight | 90.75 g/mol | 74.69 g/mol |
| Density | 5.5 g/cm3 | 6.67 g/cm3 |
| Electrical Conductivity | Semi-metallic | Semiconductor |
| Applications | Battery cathodes, catalysts, thermoelectric materials | Battery electrodes, fuel cells, ceramics, catalysts |
| Corrosion Resistance | Moderate | High |
| Thermal Stability | Stable up to 500degC | Stable up to 1000degC |
Introduction to Nickel Sulfide and Nickel Oxide
Nickel sulfide (NiS) and nickel oxide (NiO) are two primary nickel compounds widely used in industrial applications. Nickel sulfide typically forms in sulfide ores and is crucial for nickel extraction through smelting processes, while nickel oxide is often derived from laterite ores and serves as a key material in battery cathodes and ceramics. The chemical properties of nickel sulfide enable it to participate in catalytic reactions, whereas nickel oxide's stability and electrical conductivity make it essential in electronic components.
Chemical Structures and Composition Comparison
Nickel sulfide (NiS) and nickel oxide (NiO) differ significantly in chemical structure and composition; nickel sulfide consists of nickel ions bonded to sulfur atoms, forming a covalent lattice with variable stoichiometry such as Ni3S2 or NiS2. In contrast, nickel oxide features nickel ions coordinated with oxygen atoms in a cubic rock salt crystal structure, typically maintaining a consistent NiO stoichiometry. These structural differences influence their electronic properties and reactivity, with nickel sulfide exhibiting metallic conductivity while nickel oxide behaves as a p-type semiconductor.
Physical Properties: Nickel Sulfide vs Nickel Oxide
Nickel sulfide (NiS) exhibits a dark green to black crystalline appearance with a density of approximately 5.7 g/cm3 and a melting point around 1003degC, while nickel oxide (NiO) appears as a green to black powder or crystalline solid with a slightly lower density near 6.67 g/cm3 and a higher melting point of about 1955degC. Nickel sulfide is generally more electrically conductive and exhibits metallic luster, whereas nickel oxide is a non-stoichiometric p-type semiconductor with a duller, matte surface. The magnetic properties also differ significantly; nickel sulfide is paramagnetic at room temperature, whereas nickel oxide behaves as an antiferromagnetic material below its Neel temperature of 523 K.
Synthesis Methods and Industrial Production
Nickel sulfide is typically synthesized through precipitation methods using nickel salts and sulfur sources, often involving hydrothermal treatment to enhance crystallinity, while nickel oxide is primarily produced via thermal decomposition of nickel compounds such as nickel hydroxide or nickel carbonate in oxidizing atmospheres. Industrial production of nickel oxide mainly employs the roasting of nickel sulfide ores followed by leaching and calcination, whereas nickel sulfide is more commonly found as a naturally occurring mineral and refined through pyrometallurgical processes. The choice of synthesis method directly influences the material's structural properties, phase purity, and suitability for applications in catalysis or battery electrodes.
Applications in Modern Industry
Nickel sulfide is primarily utilized in the electronics industry for its excellent conductivity and magnetic properties, making it ideal for catalysts, batteries, and superconductors. Nickel oxide, known for its high thermal stability and corrosion resistance, is widely applied in ceramics, glass manufacturing, and as a component in rechargeable battery cathodes. Both compounds play crucial roles in modern industrial applications, with nickel oxide favored for energy storage solutions and nickel sulfide essential in electronic and catalytic processes.
Electrical and Magnetic Properties
Nickel sulfide exhibits lower electrical conductivity compared to nickel oxide due to its semiconducting nature, which affects its performance in electronic applications. Nickel oxide demonstrates strong p-type semiconductor behavior with higher electrical conductivity, making it suitable for electrodes and energy storage devices. Magnetically, nickel sulfide shows ferromagnetic properties with significant magnetic ordering, whereas nickel oxide is antiferromagnetic, resulting in a lack of net magnetization at room temperature.
Environmental Impact and Safety Considerations
Nickel sulfide releases sulfur compounds during processing, contributing to acid rain and posing greater environmental hazards than nickel oxide, which is more chemically stable and less reactive. Nickel sulfide dust is highly toxic and can cause respiratory issues and skin irritation, requiring stringent safety measures, while nickel oxide is considered less harmful but still requires handling precautions to prevent inhalation. Proper waste management and emission controls are critical to minimize environmental contamination and occupational exposure for both nickel compounds.
Cost and Market Availability
Nickel sulfide generally costs less than nickel oxide due to lower processing expenses and more abundant raw materials, making it a cost-effective option for industrial use. Market availability of nickel sulfide is higher in regions with established mining operations, while nickel oxide faces supply constraints linked to its more complex refining processes. Demand for nickel oxide tends to surge in high-purity applications like battery manufacturing, influencing price volatility and limited market stock compared to the more stable nickel sulfide market.
Performance in Battery and Energy Storage Technologies
Nickel sulfide offers superior electrical conductivity compared to nickel oxide, making it highly efficient for battery electrodes and energy storage applications. Nickel oxide provides excellent thermal stability and cycling durability, enhancing battery life and performance. Combining nickel sulfide's conductivity with nickel oxide's stability optimizes energy density and charge-discharge rates in advanced battery technologies.
Future Trends in Nickel Sulfide and Nickel Oxide Research
Nickel sulfide and nickel oxide exhibit distinct electrochemical properties that drive ongoing research into their applications in energy storage and catalysis. Advances in nanostructuring and doping techniques are enhancing the conductivity and catalytic efficiency of nickel sulfide, making it a promising candidate for next-generation batteries and hydrogen evolution reactions. Meanwhile, nickel oxide research emphasizes improving stability and scalability for use in supercapacitors and gas sensors, indicating a future trend toward hybrid materials combining the strengths of both compounds.
Nickel Sulfide vs Nickel Oxide Infographic
 materialdif.com
materialdif.com