Aluminum alloys offer excellent corrosion resistance, good strength-to-weight ratio, and are widely used in automotive and aerospace industries for structural components. Magnesium alloys are significantly lighter than aluminum, providing superior weight savings and enhanced vibration damping, but they tend to have lower corrosion resistance and require protective coatings. Selecting between aluminum and magnesium alloys depends on the balance between weight reduction needs and durability requirements in specific engineering applications.
Table of Comparison
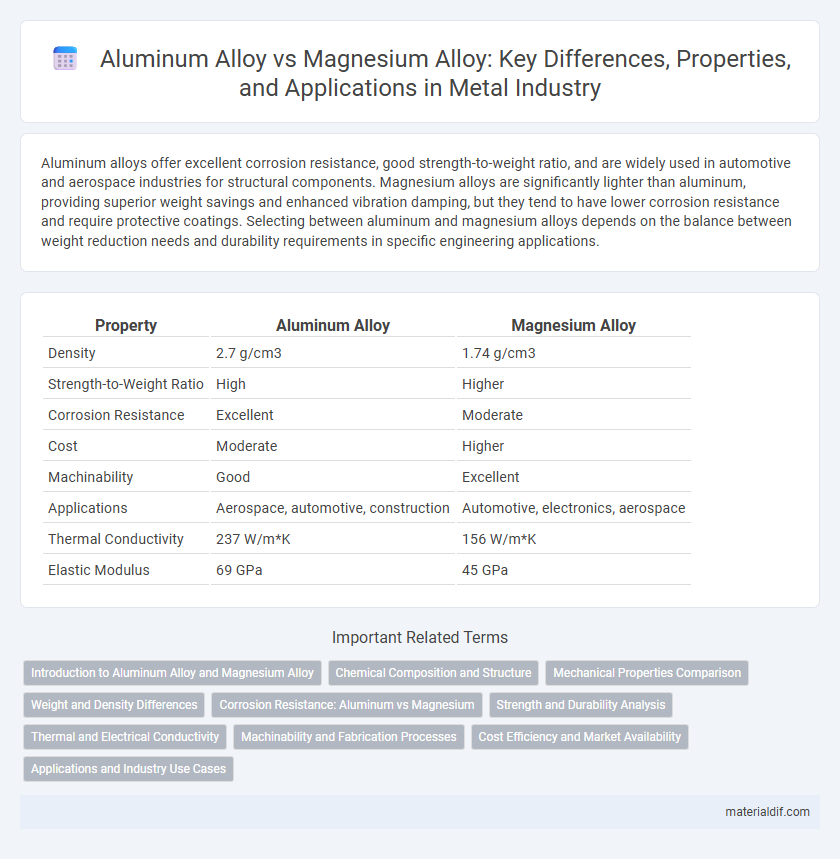
| Property | Aluminum Alloy | Magnesium Alloy |
|---|---|---|
| Density | 2.7 g/cm3 | 1.74 g/cm3 |
| Strength-to-Weight Ratio | High | Higher |
| Corrosion Resistance | Excellent | Moderate |
| Cost | Moderate | Higher |
| Machinability | Good | Excellent |
| Applications | Aerospace, automotive, construction | Automotive, electronics, aerospace |
| Thermal Conductivity | 237 W/m*K | 156 W/m*K |
| Elastic Modulus | 69 GPa | 45 GPa |
Introduction to Aluminum Alloy and Magnesium Alloy
Aluminum alloys are lightweight metals composed primarily of aluminum combined with elements such as copper, magnesium, manganese, or silicon to enhance strength and corrosion resistance. Magnesium alloys, composed mainly of magnesium and alloying elements like aluminum, zinc, or zirconium, are known for their exceptional lightness and high strength-to-weight ratio. Both alloys find extensive applications in the aerospace, automotive, and manufacturing industries due to their unique mechanical properties and corrosion resistance.
Chemical Composition and Structure
Aluminum alloys primarily consist of aluminum combined with elements like copper, silicon, magnesium, and zinc, resulting in a face-centered cubic (FCC) crystal structure that offers excellent corrosion resistance and moderate strength. Magnesium alloys, on the other hand, are mainly composed of magnesium with small amounts of aluminum, zinc, and manganese, featuring a hexagonal close-packed (HCP) structure that provides higher strength-to-weight ratio but lower corrosion resistance than aluminum alloys. The distinct crystalline structures influence mechanical properties, where aluminum alloys exhibit better ductility and magnesium alloys achieve superior lightweight performance critical in aerospace and automotive applications.
Mechanical Properties Comparison
Aluminum alloys generally offer higher tensile strength and better corrosion resistance compared to magnesium alloys, making them suitable for structural applications requiring durability. Magnesium alloys exhibit superior weight reduction due to their lower density, coupled with excellent damping capacity, which is beneficial in automotive and aerospace industries. However, magnesium alloys tend to have lower fatigue strength and are more prone to corrosion, necessitating protective coatings in mechanical applications.
Weight and Density Differences
Aluminum alloy typically has a density of around 2.7 g/cm3, making it heavier than magnesium alloy, which has a density near 1.74 g/cm3, contributing to magnesium's status as one of the lightest structural metals available. The lower density of magnesium alloys allows for significant weight reduction in automotive and aerospace applications, improving fuel efficiency and performance. Despite magnesium's lower density, aluminum alloys often offer higher strength-to-weight ratios and better corrosion resistance, influencing material selection based on specific engineering requirements.
Corrosion Resistance: Aluminum vs Magnesium
Aluminum alloys exhibit superior corrosion resistance compared to magnesium alloys due to the formation of a stable, protective oxide layer that prevents further oxidation. Magnesium alloys are more prone to galvanic corrosion and require coatings or treatments to enhance durability in harsh environments. The improved corrosion resistance of aluminum alloys makes them the preferred choice in automotive and aerospace applications where longevity and maintenance are critical.
Strength and Durability Analysis
Aluminum alloys exhibit superior corrosion resistance and tensile strength, making them ideal for applications requiring long-term durability under various environmental conditions. Magnesium alloys, while lighter and offering excellent strength-to-weight ratios, tend to have lower fatigue resistance and are more susceptible to corrosion unless properly treated. The choice between aluminum and magnesium alloys depends on balancing strength requirements with weight constraints and environmental exposure.
Thermal and Electrical Conductivity
Aluminum alloys exhibit higher electrical conductivity, typically around 35-40 MS/m, compared to magnesium alloys, which generally range from 7-10 MS/m, making aluminum more suitable for electrical applications. In terms of thermal conductivity, aluminum alloys offer superior performance, with values near 150-230 W/m*K, whereas magnesium alloys usually have lower thermal conductivity, approximately 90-120 W/m*K, affecting heat dissipation efficiency. These differences position aluminum alloys as better candidates for heat exchangers and electrical components, while magnesium alloys are favored for lightweight structural uses where thermal and electrical conductivity are less critical.
Machinability and Fabrication Processes
Aluminum alloys exhibit superior machinability compared to magnesium alloys due to their lower hardness and better thermal conductivity, allowing for faster cutting speeds and reduced tool wear during fabrication. Magnesium alloys, while lighter and offering excellent strength-to-weight ratios, present challenges such as higher tool wear and the need for specialized machining techniques to prevent ignition risks. Fabrication processes for aluminum alloys often involve conventional methods like milling and turning, whereas magnesium alloys require controlled environments and tailored machining parameters to ensure safety and maintain dimensional accuracy.
Cost Efficiency and Market Availability
Aluminum alloy offers superior cost efficiency due to its widespread availability and lower raw material expenses compared to magnesium alloy, which remains pricier and less abundant in the market. Magnesium alloy, despite its lightweight properties, incurs higher production costs and limited supply chain infrastructure, constraining its competitive pricing. Market availability of aluminum alloys supports economies of scale, making them the preferred choice for industries prioritizing affordability and consistent material sourcing.
Applications and Industry Use Cases
Aluminum alloys dominate aerospace, automotive, and packaging industries due to their excellent corrosion resistance, lightweight, and high strength-to-weight ratio. Magnesium alloys find specialized applications in electronics, automotive components, and aerospace sectors where ultra-lightweight materials and vibration damping are critical. Both alloys offer unique benefits: aluminum alloys prioritize durability and versatility, while magnesium alloys excel in reducing weight and enhancing mechanical damping performance.
Aluminum Alloy vs Magnesium Alloy Infographic
 materialdif.com
materialdif.com