Rust formation on iron occurs when moisture and oxygen react with the metal, producing a flaky, brittle compound called iron oxide that weakens the structure. In contrast, oxide scale formation is a controlled process at high temperatures, producing a dense, protective layer of stable iron oxide that prevents further corrosion. Understanding the difference between rust and oxide scale is crucial for managing iron durability in various industrial applications.
Table of Comparison
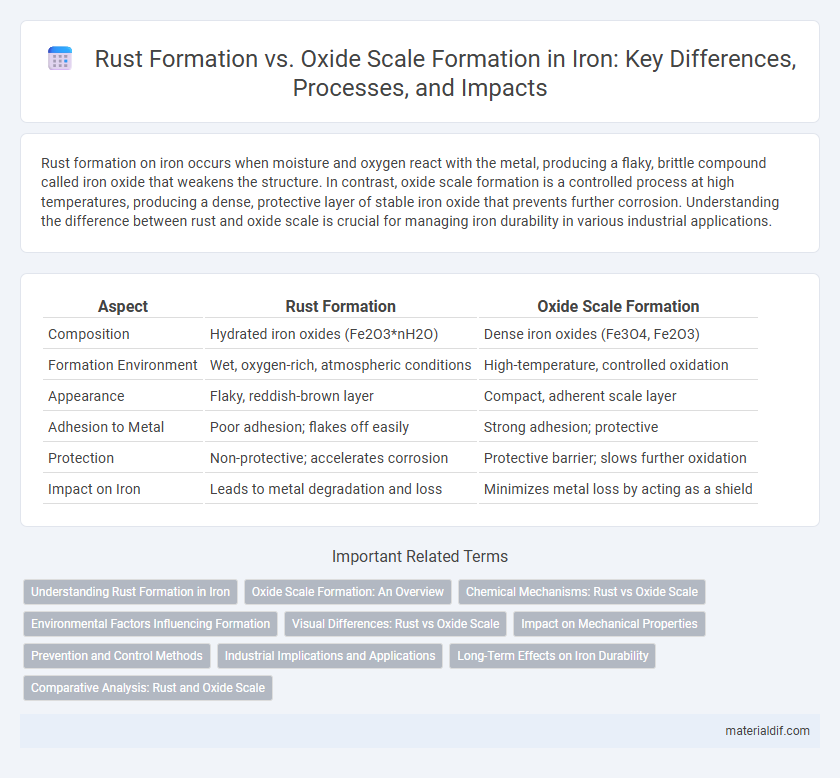
| Aspect | Rust Formation | Oxide Scale Formation |
|---|---|---|
| Composition | Hydrated iron oxides (Fe2O3*nH2O) | Dense iron oxides (Fe3O4, Fe2O3) |
| Formation Environment | Wet, oxygen-rich, atmospheric conditions | High-temperature, controlled oxidation |
| Appearance | Flaky, reddish-brown layer | Compact, adherent scale layer |
| Adhesion to Metal | Poor adhesion; flakes off easily | Strong adhesion; protective |
| Protection | Non-protective; accelerates corrosion | Protective barrier; slows further oxidation |
| Impact on Iron | Leads to metal degradation and loss | Minimizes metal loss by acting as a shield |
Understanding Rust Formation in Iron
Rust formation in iron occurs when iron reacts with oxygen and moisture, producing hydrated iron(III) oxide, a flaky and porous compound that weakens the metal's structure. Unlike oxide scale formation, which is a dense, protective layer of iron oxide formed at high temperatures, rust grows through layers and allows continued corrosion. Understanding the electrochemical process involving anodic iron dissolution and cathodic oxygen reduction is essential for preventing rust and enhancing iron durability.
Oxide Scale Formation: An Overview
Oxide scale formation on iron surfaces occurs when iron reacts with oxygen at elevated temperatures, producing a layered structure primarily composed of iron oxides such as Fe2O3 and Fe3O4. This scale acts as a protective barrier that slows further oxidation, differing from rust which is flaky and allows continued degradation. The thickness and adherence of the oxide scale depend on temperature, oxygen availability, and exposure time, influencing the material's corrosion resistance in industrial applications.
Chemical Mechanisms: Rust vs Oxide Scale
Rust formation on iron involves the electrochemical reaction between iron, oxygen, and water, producing hydrated iron(III) oxides such as Fe2O3*nH2O, which are porous and flaky, allowing further corrosion. Oxide scale formation, in contrast, results from high-temperature oxidation, forming a dense, adherent layer of iron oxides like Fe3O4 (magnetite) or FeO that acts as a protective barrier against oxygen diffusion. The key chemical mechanism difference lies in rust's aqueous environment-driven redox reactions producing unstable hydrated oxides, whereas oxide scale forms through solid-state diffusion processes at elevated temperatures yielding stable, compact oxide layers.
Environmental Factors Influencing Formation
Rust formation on iron primarily occurs in the presence of moisture and oxygen, with high humidity and acidic environments accelerating the electrochemical reactions that produce hydrated iron oxides. In contrast, oxide scale formation typically develops at elevated temperatures during processes such as steel manufacturing, where oxygen-rich atmospheres promote the growth of dense, adherent iron oxide layers. Environmental factors such as temperature, oxygen concentration, and pH levels critically influence whether iron undergoes rusting or forms protective oxide scales.
Visual Differences: Rust vs Oxide Scale
Rust formation on iron appears as a flaky, reddish-brown layer that easily flakes off, exposing the metal beneath to further corrosion. In contrast, oxide scale forms a dense, adherent, typically dark gray or bluish layer that acts as a protective barrier, preventing deeper oxidation. The visual distinction lies in rust's porous, uneven texture versus the smooth, compact appearance of oxide scale.
Impact on Mechanical Properties
Rust formation on iron typically leads to extensive material degradation, causing significant weakening of mechanical properties such as tensile strength and ductility due to porous and flaky layers. In contrast, oxide scale formation creates a more adherent, protective layer that preserves the iron's structural integrity and minimizes loss of mechanical performance. The difference in morphology and adhesion between rust and oxide scales directly influences the durability and lifespan of iron components under corrosive environments.
Prevention and Control Methods
Rust formation on iron primarily occurs due to prolonged exposure to moisture and oxygen, resulting in flaky and brittle iron oxide that weakens the metal structure. Prevention methods include applying protective coatings such as paint, galvanization with zinc, and using corrosion inhibitors that create a barrier against environmental elements. Control techniques involve regular inspection, surface cleaning to remove early rust deposits, and employing cathodic protection to reduce electrochemical reactions causing rust.
Industrial Implications and Applications
Rust formation on iron surfaces primarily results from prolonged exposure to moisture and oxygen, leading to iron oxide flakes that compromise structural integrity and increase maintenance costs in industrial settings. In contrast, oxide scale formation occurs at high temperatures during processes like steel manufacturing, producing a dense, adherent layer that can protect the metal from further oxidation but may require descaling for subsequent processing. Understanding the differences between rust and oxide scale is critical for industries such as construction, automotive, and metallurgy to optimize corrosion prevention strategies and improve material longevity.
Long-Term Effects on Iron Durability
Rust formation on iron results in flaky, porous layers that accelerate structural degradation and reduce long-term durability by promoting moisture penetration and internal corrosion. In contrast, oxide scale formation creates a dense, adherent protective barrier that slows further oxidation and preserves iron's mechanical strength over extended periods. Understanding these differences is crucial for predicting iron lifespan and implementing effective corrosion protection strategies.
Comparative Analysis: Rust and Oxide Scale
Rust formation on iron primarily occurs due to prolonged exposure to moisture and oxygen, resulting in flaky, reddish-brown iron oxide that weakens the metal structure. In contrast, oxide scale forms at high temperatures during processes like hot rolling, producing a dense, adherent layer of iron oxides (Fe3O4 and Fe2O3) that can protect the underlying metal from further oxidation. The key difference lies in rust being a porous, non-protective corrosion product, while oxide scale acts as a stable barrier improving iron's resistance to further chemical degradation.
Rust Formation vs Oxide Scale Formation Infographic
 materialdif.com
materialdif.com