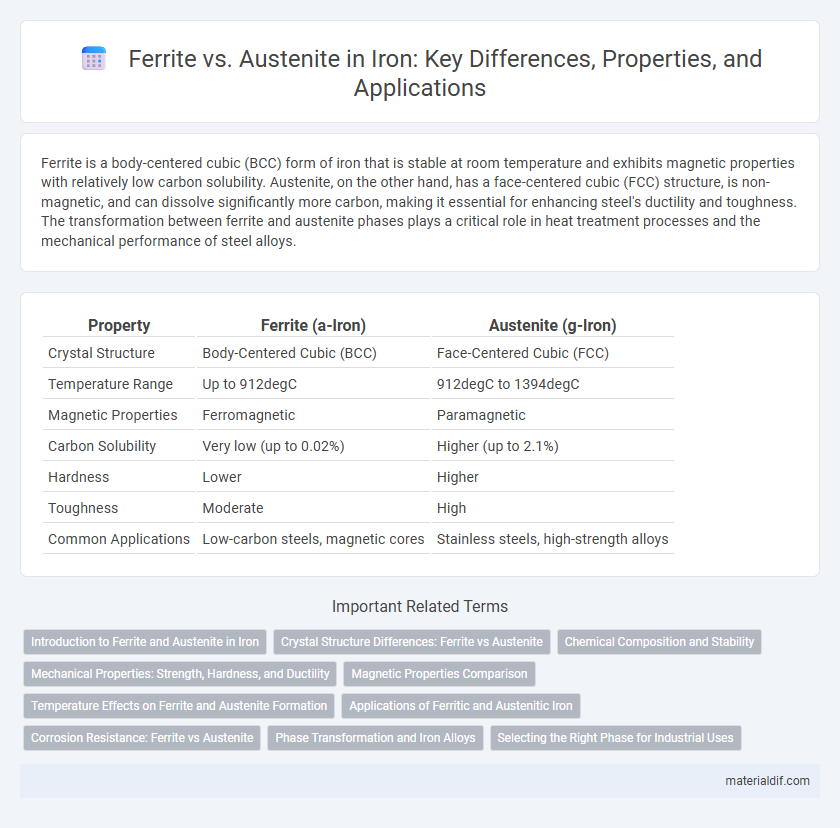
Ferrite is a body-centered cubic (BCC) form of iron that is stable at room temperature and exhibits magnetic properties with relatively low carbon solubility. Austenite, on the other hand, has a face-centered cubic (FCC) structure, is non-magnetic, and can dissolve significantly more carbon, making it essential for enhancing steel's ductility and toughness. The transformation between ferrite and austenite phases plays a critical role in heat treatment processes and the mechanical performance of steel alloys.
Table of Comparison
| Property | Ferrite (a-Iron) | Austenite (g-Iron) |
|---|---|---|
| Crystal Structure | Body-Centered Cubic (BCC) | Face-Centered Cubic (FCC) |
| Temperature Range | Up to 912degC | 912degC to 1394degC |
| Magnetic Properties | Ferromagnetic | Paramagnetic |
| Carbon Solubility | Very low (up to 0.02%) | Higher (up to 2.1%) |
| Hardness | Lower | Higher |
| Toughness | Moderate | High |
| Common Applications | Low-carbon steels, magnetic cores | Stainless steels, high-strength alloys |
Introduction to Ferrite and Austenite in Iron
Ferrite, a body-centered cubic (BCC) form of iron, is stable at room temperature and exhibits magnetic properties with low carbon solubility, making it soft and ductile. Austenite, or gamma iron, has a face-centered cubic (FCC) structure and exists at higher temperatures, accommodating more carbon atoms, which enhances its toughness and strength. The transformation between ferrite and austenite phases is critical in heat treatment processes to tailor the mechanical properties of steel.
Crystal Structure Differences: Ferrite vs Austenite
Ferrite exhibits a body-centered cubic (BCC) crystal structure that provides high magnetic permeability and lower carbon solubility, making it stable at room temperature. Austenite, characterized by a face-centered cubic (FCC) crystal structure, allows greater carbon solubility and enhanced ductility, forming primarily at higher temperatures. These distinct atomic arrangements influence mechanical properties and phase transformation behavior in steel alloys.
Chemical Composition and Stability
Ferrite is a body-centered cubic (BCC) iron phase containing low carbon content, typically less than 0.02%, which provides high magnetic permeability and mechanical stability at room temperature. Austenite, a face-centered cubic (FCC) phase, stabilizes at higher temperatures with carbon solubility extending up to 2.1%, enhancing ductility and toughness. Stability of ferrite predominates below 912degC, transforming into stable austenite as temperature rises, influenced by alloying elements like nickel and manganese that expand the austenite phase field.
Mechanical Properties: Strength, Hardness, and Ductility
Ferrite, a body-centered cubic (BCC) form of iron, exhibits lower strength and hardness but higher ductility compared to austenite, which has a face-centered cubic (FCC) structure. Austenite provides superior toughness and elevated temperature strength due to its FCC lattice facilitating more slip systems, enhancing deformation capabilities without fracture. The difference in crystal structure directly influences their mechanical properties, making ferrite suitable for applications requiring formability, while austenite is preferred where strength and hardness are critical.
Magnetic Properties Comparison
Ferrite exhibits strong ferromagnetic properties due to its body-centered cubic (BCC) crystal structure, making it highly magnetic and suitable for electromagnetic applications. Austenite, with a face-centered cubic (FCC) structure, is generally paramagnetic and lacks the magnetic permeability found in ferrite. This fundamental difference in crystal structures directly influences their magnetic behavior, with ferrite being widely used in transformers and inductors, while austenite is preferred in non-magnetic stainless steels.
Temperature Effects on Ferrite and Austenite Formation
Ferrite forms at lower temperatures, typically below 912degC, characterized by a body-centered cubic (BCC) crystal structure that provides high magnetic permeability and low carbon solubility. Austenite develops at higher temperatures between 912degC and 1394degC, featuring a face-centered cubic (FCC) structure with enhanced carbon solubility and improved ductility. Temperature significantly dictates phase stability in iron, influencing mechanical properties and microstructural transformations in steel manufacturing.
Applications of Ferritic and Austenitic Iron
Ferritic iron, characterized by a body-centered cubic (BCC) crystal structure, is widely used in automotive exhaust systems and appliances due to its excellent magnetic properties and good corrosion resistance at moderate temperatures. Austenitic iron, with a face-centered cubic (FCC) structure and high chromium and nickel content, is the preferred choice in chemical processing equipment, food industry tools, and cryogenic applications because of its superior corrosion resistance and outstanding ductility. Both ferritic and austenitic irons play crucial roles in industrial engineering, with ferritic grades favored for their cost-effectiveness and magnetic properties, while austenitic variants dominate where enhanced toughness and corrosion resistance are essential.
Corrosion Resistance: Ferrite vs Austenite
Ferrite and Austenite differ significantly in corrosion resistance, with Austenitic stainless steels exhibiting superior resistance due to their higher chromium and nickel content, which forms a stable passive oxide layer. Ferritic stainless steels, while less corrosion-resistant than austenitic types, still offer good resistance to stress corrosion cracking and chloride-induced corrosion in mildly corrosive environments. The magnetic properties of Ferrite contrast with the non-magnetic nature of Austenite, influencing their application based on corrosion exposure and mechanical requirements.
Phase Transformation and Iron Alloys
Ferrite and austenite represent two crucial phases in iron alloys characterized by distinct crystal structures, with ferrite having a body-centered cubic (BCC) structure and austenite exhibiting a face-centered cubic (FCC) structure. Phase transformation between ferrite and austenite occurs primarily through heating and cooling cycles, influencing mechanical properties and alloy performance; ferrite is stable at lower temperatures while austenite forms at higher temperatures typically above 912degC. Alloying elements such as carbon, manganese, and nickel play significant roles in stabilizing these phases, enhancing strength, ductility, and corrosion resistance in steels through controlled phase transformation.
Selecting the Right Phase for Industrial Uses
Ferrite, a body-centered cubic (BCC) phase of iron, offers high magnetic permeability and good ductility, making it ideal for applications such as electrical transformers and soft magnetic materials. Austenite, characterized by its face-centered cubic (FCC) structure, provides superior toughness and corrosion resistance, widely used in stainless steels and cryogenic environments. Selecting the right phase depends on the desired mechanical properties and environmental conditions, with ferrite excelling in low-temperature magnetics and austenite favored for strength and corrosion resistance under demanding industrial settings.
Ferrite vs Austenite Infographic
 materialdif.com
materialdif.com