Vitreous carbon exhibits a dense, non-porous structure with high chemical resistance and thermal stability, making it ideal for applications requiring inert and robust materials. Porous carbon, characterized by a high surface area and interconnected pore networks, excels in adsorption, catalysis, and energy storage due to enhanced mass transport and active site accessibility. Selecting between vitreous and porous carbon depends on whether mechanical strength or surface reactivity is prioritized in the intended application.
Table of Comparison
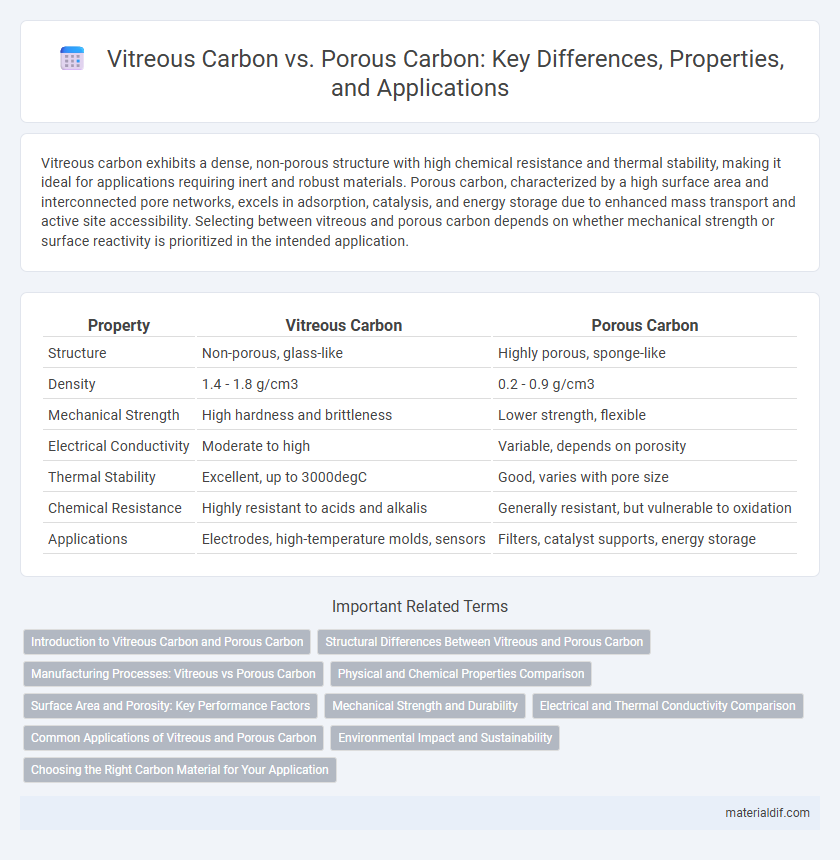
| Property | Vitreous Carbon | Porous Carbon |
|---|---|---|
| Structure | Non-porous, glass-like | Highly porous, sponge-like |
| Density | 1.4 - 1.8 g/cm3 | 0.2 - 0.9 g/cm3 |
| Mechanical Strength | High hardness and brittleness | Lower strength, flexible |
| Electrical Conductivity | Moderate to high | Variable, depends on porosity |
| Thermal Stability | Excellent, up to 3000degC | Good, varies with pore size |
| Chemical Resistance | Highly resistant to acids and alkalis | Generally resistant, but vulnerable to oxidation |
| Applications | Electrodes, high-temperature molds, sensors | Filters, catalyst supports, energy storage |
Introduction to Vitreous Carbon and Porous Carbon
Vitreous carbon is a non-graphitizing, glass-like carbon material characterized by its high chemical resistance, thermal stability, and impermeability, often used in electrochemical applications and high-temperature environments. Porous carbon, in contrast, features a network of interconnected pores that provide a high surface area, making it ideal for adsorption, catalysis, and energy storage applications such as supercapacitors and gas purification. Both materials exhibit distinct microstructures and properties that determine their suitability for specific industrial and scientific uses.
Structural Differences Between Vitreous and Porous Carbon
Vitreous carbon exhibits a non-porous, glass-like structure characterized by a highly ordered, dense arrangement of carbon atoms, resulting in exceptional chemical resistance and high electrical conductivity. Porous carbon features a network of interconnected pores with variable pore sizes, offering a large surface area that enhances adsorption and catalytic activity but reduces mechanical strength. The structural difference between these two forms lies in the atomic organization and pore architecture, where vitreous carbon is compact and impermeable, while porous carbon contains extensive voids facilitating diffusion and surface reactions.
Manufacturing Processes: Vitreous vs Porous Carbon
Vitreous carbon is produced through the pyrolysis of polymer precursors at high temperatures under inert atmospheres, resulting in a dense, glass-like structure with minimal porosity. Porous carbon is typically manufactured using templating methods, activation processes, or incorporation of pore-forming agents during carbonization to create a network of micro- and mesopores. The choice of manufacturing process directly influences the carbon's structural properties, surface area, and applications in areas such as electrochemistry and filtration.
Physical and Chemical Properties Comparison
Vitreous carbon is a non-porous, hard material characterized by a smooth surface, high chemical resistance, and excellent thermal stability, making it ideal for applications requiring impermeability and durability. Porous carbon exhibits a high surface area with interconnected pores, enhancing its adsorption capacity and reactivity but reducing mechanical strength and chemical resistance. The key differences lie in vitreous carbon's dense, glass-like structure versus the highly porous, reactive nature of porous carbon, dictating their distinct uses in electrochemistry, filtration, and catalysis.
Surface Area and Porosity: Key Performance Factors
Vitreous carbon exhibits low surface area and negligible porosity, offering high chemical resistance and mechanical strength ideal for applications requiring dense, non-porous materials. In contrast, porous carbon features high surface area and well-developed pore structures, enhancing adsorption capacity and catalytic activity in energy storage and filtration systems. The distinct differences in surface area and porosity between vitreous and porous carbon critically influence their performance and suitability across various industrial uses.
Mechanical Strength and Durability
Vitreous carbon exhibits superior mechanical strength and higher durability compared to porous carbon due to its dense, non-porous microstructure that resists deformation and wear under mechanical stress. Porous carbon, characterized by interconnected voids, offers lower mechanical robustness but better accommodates thermal expansion and chemical interactions in applications requiring lightweight materials. The choice between vitreous and porous carbon depends on specific demands for strength retention and longevity under harsh operational environments.
Electrical and Thermal Conductivity Comparison
Vitreous carbon exhibits superior electrical conductivity due to its dense, non-porous microstructure, facilitating efficient electron flow compared to porous carbon, which suffers from interrupted conductive pathways. Thermal conductivity in vitreous carbon is also higher, as its compact atomic arrangement allows rapid phonon transfer, unlike porous carbon where voids impede heat conduction. Consequently, vitreous carbon is preferred in high-performance applications requiring reliable electrical and thermal conduction.
Common Applications of Vitreous and Porous Carbon
Vitreous carbon is widely used in electrochemical electrodes, high-temperature crucibles, and chemical-resistant components due to its low porosity, high chemical stability, and excellent electrical conductivity. Porous carbon finds common applications in filtration systems, catalyst supports, energy storage devices like supercapacitors, and gas adsorption because of its high surface area and tunable pore structure. Both types of carbon materials are integral in energy conversion, environmental remediation, and chemical processing industries for their distinct physical and chemical properties.
Environmental Impact and Sustainability
Vitreous carbon exhibits high chemical resistance and low porosity, resulting in minimal environmental contamination and enhanced durability for sustainable applications. Porous carbon, with its high surface area and tunable porosity, supports efficient pollutant adsorption and carbon capture but may require energy-intensive manufacturing processes impacting overall sustainability. Selecting between vitreous and porous carbon depends on balancing environmental impact with functional benefits for targeted eco-friendly solutions.
Choosing the Right Carbon Material for Your Application
Vitreous carbon offers high chemical resistance, low density, and excellent thermal stability, making it ideal for applications requiring inertness and structural integrity, such as electrodes in electrochemistry or high-temperature crucibles. Porous carbon provides a high surface area with tunable pore sizes, enhancing adsorption, catalysis, and energy storage performance, suitable for applications like supercapacitors and filtration systems. Selecting between vitreous and porous carbon depends on the need for mechanical strength and chemical inertness versus surface area and permeability tailored to specific industrial or research requirements.
Vitreous Carbon vs Porous Carbon Infographic
 materialdif.com
materialdif.com