Graphite consists of multiple layers of carbon atoms arranged in a hexagonal lattice, providing excellent conductivity and lubrication properties. Graphene, a single layer of carbon atoms, exhibits extraordinary strength, flexibility, and electrical conductivity due to its two-dimensional structure. The unique atomic arrangement in graphene results in superior mechanical and electronic characteristics compared to the multi-layered form of graphite.
Table of Comparison
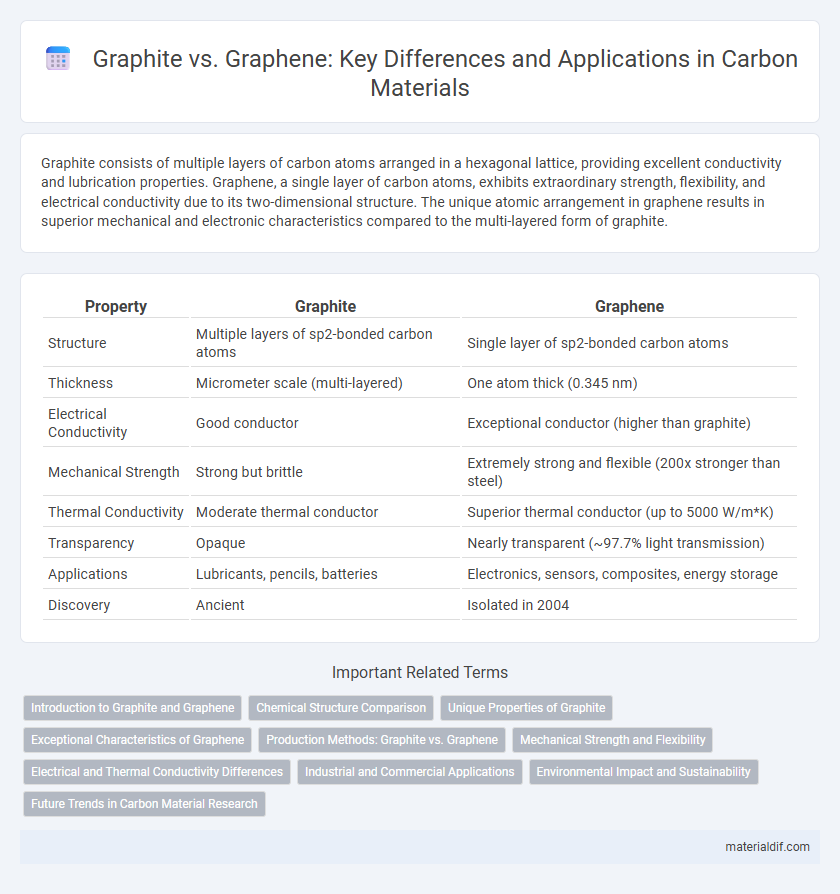
| Property | Graphite | Graphene |
|---|---|---|
| Structure | Multiple layers of sp2-bonded carbon atoms | Single layer of sp2-bonded carbon atoms |
| Thickness | Micrometer scale (multi-layered) | One atom thick (0.345 nm) |
| Electrical Conductivity | Good conductor | Exceptional conductor (higher than graphite) |
| Mechanical Strength | Strong but brittle | Extremely strong and flexible (200x stronger than steel) |
| Thermal Conductivity | Moderate thermal conductor | Superior thermal conductor (up to 5000 W/m*K) |
| Transparency | Opaque | Nearly transparent (~97.7% light transmission) |
| Applications | Lubricants, pencils, batteries | Electronics, sensors, composites, energy storage |
| Discovery | Ancient | Isolated in 2004 |
Introduction to Graphite and Graphene
Graphite is a naturally occurring form of carbon composed of multiple layers of carbon atoms arranged in a hexagonal lattice, known for its use in pencils and lubricants due to its softness and electrical conductivity. Graphene, a single layer of carbon atoms tightly packed in a two-dimensional honeycomb lattice, exhibits remarkable strength, electrical conductivity, and thermal properties far surpassing those of bulk graphite. These unique characteristics of graphene make it a revolutionary material for advanced electronics, energy storage, and composite materials.
Chemical Structure Comparison
Graphite consists of multiple layers of carbon atoms arranged in a hexagonal lattice, where each layer is held together by weak van der Waals forces, allowing easy separation. Graphene is a single atomic layer of carbon atoms bonded in a two-dimensional honeycomb lattice, exhibiting sp2 hybridization with strong covalent bonds within the plane. The distinct chemical structures result in graphene's exceptional electrical conductivity and mechanical strength compared to graphite.
Unique Properties of Graphite
Graphite exhibits exceptional electrical conductivity due to its layered crystal structure, allowing electrons to move freely within planes of carbon atoms arranged in hexagonal lattices. Its excellent thermal stability and high lubricity make it an ideal material for applications ranging from electrodes in batteries to lubricants in machinery. Unlike graphene, graphite's bulk form provides mechanical robustness and ease of fabrication for industrial use.
Exceptional Characteristics of Graphene
Graphene exhibits exceptional characteristics including remarkable electrical conductivity, mechanical strength over 200 times greater than steel, and outstanding thermal conductivity exceeding 5,000 W/mK. Its single-atom-thick lattice of carbon atoms arranged in a hexagonal pattern enables extraordinary flexibility and transparency, surpassing graphite's multilayered structure. These unique properties position graphene as a revolutionary material for advanced applications in electronics, energy storage, and nanotechnology.
Production Methods: Graphite vs. Graphene
Graphite is primarily produced through mining natural deposits or synthetic graphitization of carbon-rich materials at high temperatures above 2500degC. Graphene synthesis involves advanced techniques such as chemical vapor deposition (CVD), mechanical exfoliation, and liquid-phase exfoliation to isolate single or few layers of carbon atoms from graphite. The precise control in graphene production methods enables scalable manufacture for applications in electronics, energy storage, and composites, unlike the bulk extraction process used for graphite.
Mechanical Strength and Flexibility
Graphene exhibits exceptional mechanical strength, with a tensile strength approximately 200 times greater than steel, due to its single-layer carbon atom structure arranged in a hexagonal lattice. In contrast, graphite consists of multiple layers of graphene stacked together, resulting in weaker interlayer bonding and reduced overall strength. Graphene also offers superior flexibility, able to bend without breaking, whereas graphite's layered structure makes it more brittle and less adaptable to deformation.
Electrical and Thermal Conductivity Differences
Graphene exhibits significantly higher electrical conductivity than graphite due to its single-layer, two-dimensional structure of carbon atoms, allowing electrons to move with minimal resistance. Thermal conductivity in graphene reaches values around 5000 W/mK, surpassing graphite's typical range of 100-400 W/mK, attributed to reduced phonon scattering in its atomically thin lattice. These differences make graphene superior for applications requiring efficient heat dissipation and electrical transport, such as in advanced electronics and thermal management systems.
Industrial and Commercial Applications
Graphite, characterized by its layered structure and high electrical conductivity, is widely used in industrial applications such as electrodes in electric arc furnaces, lubricants, and refractory materials. Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, exhibits exceptional strength, flexibility, and superior electrical and thermal conductivity, driving its adoption in advanced electronics, energy storage devices like supercapacitors and batteries, and high-performance composite materials. The distinct properties of graphene enable enhanced performance and miniaturization in commercial products compared to traditional graphite-based solutions.
Environmental Impact and Sustainability
Graphene, derived from graphite, offers superior environmental benefits due to its high efficiency in energy storage and conductivity, reducing the need for resource-intensive materials. Graphite mining, however, poses sustainability challenges including habitat disruption and significant carbon emissions. Advances in graphene production methods, such as green synthesis and recycling of graphite waste, aim to minimize environmental impact and promote sustainable use of carbon materials.
Future Trends in Carbon Material Research
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, exhibits exceptional electrical conductivity, mechanical strength, and flexibility, outpacing traditional graphite in potential applications. Future trends in carbon material research emphasize scalable production methods for high-quality graphene to enable advances in electronics, energy storage, and composite materials. Innovations such as graphene-based supercapacitors and flexible electronics reflect the growing shift from bulk graphite toward graphene-centric technologies in next-generation carbon materials.
Graphite vs Graphene Infographic
 materialdif.com
materialdif.com