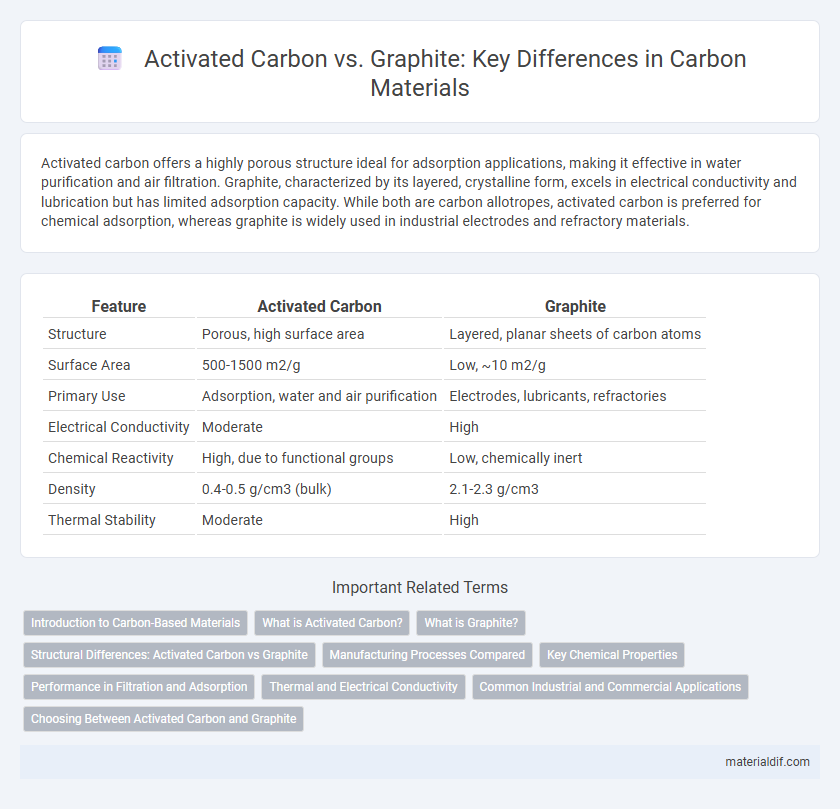
Activated carbon offers a highly porous structure ideal for adsorption applications, making it effective in water purification and air filtration. Graphite, characterized by its layered, crystalline form, excels in electrical conductivity and lubrication but has limited adsorption capacity. While both are carbon allotropes, activated carbon is preferred for chemical adsorption, whereas graphite is widely used in industrial electrodes and refractory materials.
Table of Comparison
| Feature | Activated Carbon | Graphite |
|---|---|---|
| Structure | Porous, high surface area | Layered, planar sheets of carbon atoms |
| Surface Area | 500-1500 m2/g | Low, ~10 m2/g |
| Primary Use | Adsorption, water and air purification | Electrodes, lubricants, refractories |
| Electrical Conductivity | Moderate | High |
| Chemical Reactivity | High, due to functional groups | Low, chemically inert |
| Density | 0.4-0.5 g/cm3 (bulk) | 2.1-2.3 g/cm3 |
| Thermal Stability | Moderate | High |
Introduction to Carbon-Based Materials
Activated carbon features a highly porous structure with extensive surface area, making it ideal for adsorption and filtration applications, while graphite possesses a layered, crystalline form contributing to excellent electrical conductivity and lubrication properties. Both activated carbon and graphite are allotropes of carbon distinguished by their unique physical structures and functional uses in industrial, environmental, and energy storage fields. Their contrasting characteristics stem from the sp2 hybridization in graphite creating planar sheets, versus the amorphous, irregular texture in activated carbon enhancing adsorption capabilities.
What is Activated Carbon?
Activated carbon is a highly porous form of carbon characterized by extensive surface area and microporosity, making it ideal for adsorption applications in water purification, air filtration, and chemical processing. It is produced by treating carbon-rich materials such as coconut shells, wood, or coal with heat and chemical agents to create a network of tiny pores. Unlike graphite, which is crystalline and used mainly in batteries and lubricants, activated carbon's key function lies in trapping contaminants due to its large internal surface.
What is Graphite?
Graphite is a crystalline form of carbon characterized by its layered structure of hexagonal carbon atoms, providing excellent electrical conductivity and thermal stability. Unlike activated carbon, which is highly porous and used primarily for adsorption and filtration, graphite's dense lattice makes it ideal for applications in batteries, lubricants, and electrodes. Its unique combination of strength, conductivity, and chemical inertness distinguishes graphite in various industrial and technological uses.
Structural Differences: Activated Carbon vs Graphite
Activated carbon exhibits a highly porous, amorphous structure with a vast surface area ideal for adsorption, while graphite consists of a well-ordered crystalline lattice arranged in hexagonal layers of carbon atoms. The irregular, disordered framework of activated carbon enhances its reactivity and capacity for trapping impurities, contrasting with graphite's layered planar sheets held together by van der Waals forces, which confer electrical conductivity and lubricity. These structural distinctions critically influence their applications, with activated carbon favored in filtration and purification and graphite utilized in electrodes and refractory materials.
Manufacturing Processes Compared
Activated carbon production involves the carbonization of raw materials like coconut shells or coal, followed by activation through physical or chemical methods to create a porous structure ideal for adsorption. In contrast, graphite manufacturing requires the high-temperature graphitization of carbon-rich materials such as petroleum coke, resulting in a crystalline, layered structure with high electrical conductivity. The distinct manufacturing processes dictate their unique properties and applications, with activated carbon prioritized for filtration and purification, whereas graphite serves critical roles in electrodes and lubricants.
Key Chemical Properties
Activated carbon exhibits a highly porous structure with a large surface area, making it exceptionally effective for adsorption due to its abundant reactive hydroxyl, carboxyl, and carbonyl functional groups. Graphite consists of layers of sp2-bonded carbon atoms arranged in a hexagonal lattice, giving it excellent electrical conductivity and chemical stability but limited surface area for adsorption. The distinct chemical properties of activated carbon enable it to interact with various gases and liquids, whereas graphite's structure favors applications requiring electrical conductivity and thermal resistance.
Performance in Filtration and Adsorption
Activated carbon exhibits superior adsorption capacity due to its highly porous structure, enabling efficient removal of contaminants from air and water. Graphite shows limited filtration performance but excels in electrical conductivity applications rather than adsorption. The microporous nature of activated carbon makes it the preferred material for high-performance filtration and pollutant capture.
Thermal and Electrical Conductivity
Activated carbon exhibits lower thermal and electrical conductivity compared to graphite due to its amorphous structure and high porosity, which disrupt electron flow. Graphite's crystalline lattice with delocalized electrons enables superior electrical and thermal conductivities, making it ideal for applications requiring efficient heat dissipation and electrical conduction. The intrinsic anisotropy in graphite further enhances conductivity along the planar layers, contrasting sharply with the insulating behavior of activated carbon.
Common Industrial and Commercial Applications
Activated carbon is widely used in water purification, air filtration, and chemical processing due to its high surface area and porous structure, enabling efficient adsorption of contaminants. Graphite finds common applications in electrodes for batteries, lubricants, and refractories because of its excellent electrical conductivity and thermal stability. Both materials serve distinct roles across industries, with activated carbon dominating filtration processes and graphite excelling in electrical and high-temperature environments.
Choosing Between Activated Carbon and Graphite
Activated carbon offers superior adsorption capabilities due to its high surface area and porous structure, making it ideal for filtration and purification applications. Graphite, characterized by its excellent electrical conductivity and thermal stability, is preferred in applications like electrodes and lubricants. Selecting between activated carbon and graphite depends on whether the primary requirement is adsorption efficiency or electrical and thermal properties.
Activated Carbon vs Graphite Infographic
 materialdif.com
materialdif.com