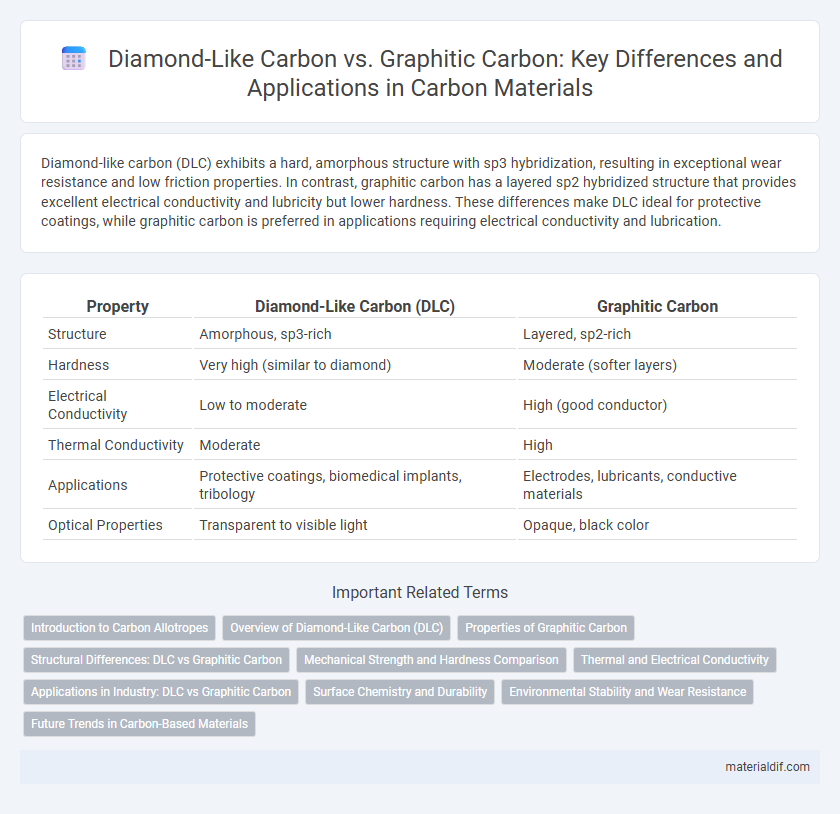
Diamond-like carbon (DLC) exhibits a hard, amorphous structure with sp3 hybridization, resulting in exceptional wear resistance and low friction properties. In contrast, graphitic carbon has a layered sp2 hybridized structure that provides excellent electrical conductivity and lubricity but lower hardness. These differences make DLC ideal for protective coatings, while graphitic carbon is preferred in applications requiring electrical conductivity and lubrication.
Table of Comparison
| Property | Diamond-Like Carbon (DLC) | Graphitic Carbon |
|---|---|---|
| Structure | Amorphous, sp3-rich | Layered, sp2-rich |
| Hardness | Very high (similar to diamond) | Moderate (softer layers) |
| Electrical Conductivity | Low to moderate | High (good conductor) |
| Thermal Conductivity | Moderate | High |
| Applications | Protective coatings, biomedical implants, tribology | Electrodes, lubricants, conductive materials |
| Optical Properties | Transparent to visible light | Opaque, black color |
Introduction to Carbon Allotropes
Diamond-like carbon (DLC) and graphitic carbon represent two distinct allotropes of carbon, each exhibiting unique atomic structures and properties. DLC features an amorphous carbon structure with a high proportion of sp3 bonds, resulting in exceptional hardness and chemical resistance, while graphitic carbon is characterized by sp2 bonded layers with delocalized electrons, providing excellent electrical conductivity and lubricity. These contrasting allotropes highlight carbon's versatile bonding configurations and its wide-ranging applications in advanced materials science.
Overview of Diamond-Like Carbon (DLC)
Diamond-like carbon (DLC) is an amorphous carbon material characterized by a mixture of sp3 and sp2 hybridized bonds, providing exceptional hardness, low friction, and chemical inertness. DLC coatings exhibit superior wear resistance and biocompatibility compared to graphitic carbon, making them ideal for protective surfaces in biomedical implants, automotive components, and electronics. The tunable properties of DLC arise from its unique microstructure, bridging the gap between diamond's hardness and graphite's lubricity.
Properties of Graphitic Carbon
Graphitic carbon exhibits a layered structure with sp2 hybridized carbon atoms arranged in hexagonal lattices, resulting in excellent electrical conductivity and lubricating properties. Its anisotropic nature provides high thermal conductivity along the planes while maintaining chemical stability in various environments. Unlike diamond-like carbon, graphitic carbon is softer and more flexible, making it suitable for applications requiring conductive coatings and solid lubricants.
Structural Differences: DLC vs Graphitic Carbon
Diamond-like carbon (DLC) exhibits a predominantly sp3 hybridized carbon structure, resulting in a tetrahedral network that provides exceptional hardness and chemical inertness. In contrast, graphitic carbon consists mainly of sp2 hybridized carbon atoms arranged in planar hexagonal sheets with delocalized pi electrons, lending it electrical conductivity and lubricating properties. These structural differences define DLC's amorphous, dense matrix versus the ordered, layered arrangement characteristic of graphitic carbon.
Mechanical Strength and Hardness Comparison
Diamond-like carbon (DLC) exhibits superior mechanical strength and hardness compared to graphitic carbon due to its predominantly sp3 hybridized carbon atoms forming a dense, tetrahedral network. Graphitic carbon, characterized by sp2 hybridization and layered planar structures, offers lower hardness and is more prone to shear and deformation under mechanical stress. The intrinsic hardness of DLC reaches values close to natural diamond, making it ideal for protective coatings, while graphitic carbon's weaker van der Waals forces between layers result in significantly reduced mechanical robustness.
Thermal and Electrical Conductivity
Diamond-like carbon exhibits exceptionally high thermal conductivity due to its strong sp3 carbon bonds, facilitating efficient heat transfer. In contrast, graphitic carbon demonstrates superior electrical conductivity attributed to its sp2 bonding and delocalized electrons within the graphene layers. These distinct bonding structures define their specialized applications in electronics and thermal management systems.
Applications in Industry: DLC vs Graphitic Carbon
Diamond-like carbon (DLC) exhibits exceptional hardness, low friction, and chemical inertness, making it ideal for protective coatings in automotive engines, cutting tools, and biomedical implants. Graphitic carbon is prized for its excellent electrical conductivity and lubricating properties, widely utilized in batteries, electrodes, and industrial lubricants. Both materials enhance performance and durability across sectors, with DLC favored in wear-resistant applications and graphitic carbon dominating energy storage and lubrication technologies.
Surface Chemistry and Durability
Diamond-like carbon (DLC) exhibits a predominantly sp3 hybridized structure, resulting in exceptional hardness, chemical inertness, and superior resistance to wear and corrosion compared to graphitic carbon, which is primarily composed of sp2 bonds. The surface chemistry of DLC offers low friction coefficients and hydrophobic properties, enhancing its durability in harsh environments, whereas graphitic carbon surfaces are more reactive and prone to oxidation and abrasion. These characteristics make DLC an ideal choice for protective coatings, while graphitic carbon is often preferred for applications requiring electrical conductivity and catalytic activity.
Environmental Stability and Wear Resistance
Diamond-like carbon (DLC) exhibits superior environmental stability due to its amorphous structure and high sp3 bonding, which enhances resistance to oxidation and chemical degradation compared to graphitic carbon. Graphitic carbon, characterized by layered sp2 bonding, is more prone to oxidation and environmental wear under harsh conditions. DLC's exceptional wear resistance and low friction coefficient make it ideal for coatings in extreme environments, outperforming the softer, more brittle graphitic layers.
Future Trends in Carbon-Based Materials
Diamond-like carbon (DLC) and graphitic carbon represent two pivotal forms in the evolution of carbon-based materials, each offering unique mechanical and electronic properties. Future trends emphasize integrating DLC's exceptional hardness and low friction with graphitic carbon's electrical conductivity and thermal stability to develop multifunctional coatings and flexible electronics. Emerging applications focus on energy storage, nanoelectronics, and biocompatible surfaces, driving advancements through hybrid carbon nanostructures and enhanced synthesis techniques.
Diamond-like carbon vs Graphitic carbon Infographic
 materialdif.com
materialdif.com