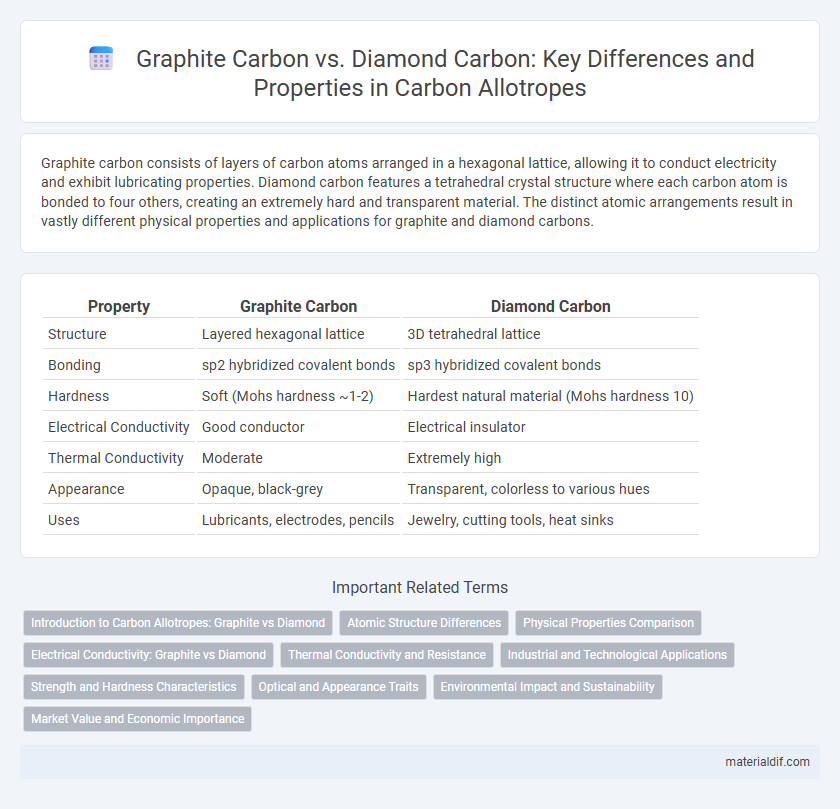
Graphite carbon consists of layers of carbon atoms arranged in a hexagonal lattice, allowing it to conduct electricity and exhibit lubricating properties. Diamond carbon features a tetrahedral crystal structure where each carbon atom is bonded to four others, creating an extremely hard and transparent material. The distinct atomic arrangements result in vastly different physical properties and applications for graphite and diamond carbons.
Table of Comparison
| Property | Graphite Carbon | Diamond Carbon |
|---|---|---|
| Structure | Layered hexagonal lattice | 3D tetrahedral lattice |
| Bonding | sp2 hybridized covalent bonds | sp3 hybridized covalent bonds |
| Hardness | Soft (Mohs hardness ~1-2) | Hardest natural material (Mohs hardness 10) |
| Electrical Conductivity | Good conductor | Electrical insulator |
| Thermal Conductivity | Moderate | Extremely high |
| Appearance | Opaque, black-grey | Transparent, colorless to various hues |
| Uses | Lubricants, electrodes, pencils | Jewelry, cutting tools, heat sinks |
Introduction to Carbon Allotropes: Graphite vs Diamond
Graphite and diamond are two prominent allotropes of carbon, distinguished by their unique atomic structures and physical properties. Graphite features a layered hexagonal lattice with sp2 hybridization, allowing electrons to move freely between layers, resulting in electrical conductivity and lubricating properties. Diamond, on the other hand, possesses a rigid tetrahedral sp3 bonded lattice, making it the hardest natural material with exceptional thermal conductivity and optical transparency.
Atomic Structure Differences
Graphite carbon consists of layers of hexagonally arranged carbon atoms bonded by strong covalent bonds within each plane but weak van der Waals forces between layers, allowing easy slippage and making it a good lubricant. Diamond carbon features a tetrahedral atomic structure where each carbon atom forms strong covalent bonds with four neighbors, creating an extremely rigid and three-dimensional network. These atomic structure differences result in graphite's electrical conductivity and softness versus diamond's exceptional hardness and electrical insulation.
Physical Properties Comparison
Graphite carbon exhibits a layered structure with weak van der Waals forces between planes, resulting in excellent electrical conductivity and a soft, slippery texture. Diamond carbon features a tetrahedral crystal lattice, making it incredibly hard, transparent, and an electrical insulator. The contrasting physical properties arise from the distinct atomic bonding arrangements in graphite's sp2 hybridization versus diamond's sp3 hybridization.
Electrical Conductivity: Graphite vs Diamond
Graphite exhibits high electrical conductivity due to its layered structure with free-moving electrons within the planar sheets of sp2-hybridized carbon atoms, enabling efficient electron flow. In contrast, diamond's sp3-hybridized tetrahedral lattice forms a strong covalent network with no free electrons, resulting in it being an excellent electrical insulator. The stark difference in atomic bonding and crystal structure between graphite and diamond directly impacts their respective electrical conductivities.
Thermal Conductivity and Resistance
Graphite carbon exhibits high thermal conductivity along its planar layers, enabling efficient heat transfer in the a-b plane, while showing poor conductivity perpendicular to these layers. In contrast, diamond carbon features exceptional isotropic thermal conductivity, surpassing most materials due to its strong covalent bonding and rigid crystal lattice. Despite diamond's superior thermal conductivity, graphite demonstrates greater thermal resistance perpendicular to its layers, making their heat transfer properties highly anisotropic and dependent on crystal orientation.
Industrial and Technological Applications
Graphite carbon's layered structure makes it ideal for industrial applications like lubricants, electrodes, and battery anodes, leveraging its electrical conductivity and thermal resistance. Diamond carbon, characterized by its crystal lattice, excels in cutting, drilling tools, and semiconductor technology due to its exceptional hardness and thermal conductivity. Both allotropes of carbon are integral to advancing industrial and technological fields through their distinct physical properties.
Strength and Hardness Characteristics
Graphite carbon exhibits a layered, planar structure with weak van der Waals forces between layers, resulting in low hardness but excellent lubricating properties, whereas diamond carbon has a tetrahedral crystal lattice with strong covalent bonds in all directions, giving it the highest known hardness and exceptional strength. The hardness of diamond, rated 10 on the Mohs scale, far exceeds graphite's value of about 1-2, making diamond ideal for industrial cutting, drilling, and abrasive applications. Graphite's anisotropic mechanical properties contrast sharply with diamond's isotropic rigidity and durability, confirming diamond's superiority in strength and hardness metrics.
Optical and Appearance Traits
Graphite carbon exhibits a metallic luster with a dark gray to black appearance, characterized by its opaque and opaque, reflective surface that absorbs most visible light. Diamond carbon shows exceptional optical clarity and brilliance due to its strong light dispersion and high refractive index, resulting in transparency and a sparkling white or colorless appearance. These contrasting optical traits arise from the differences in their crystal structures and bonding, with graphite's layered structure causing light absorption and diamond's tetrahedral lattice enabling maximum light reflection and refraction.
Environmental Impact and Sustainability
Graphite carbon exhibits a lower environmental impact compared to diamond carbon due to its abundant natural occurrence and less energy-intensive extraction processes. Diamond carbon, especially synthetic diamonds, requires high-pressure, high-temperature methods or chemical vapor deposition, leading to significant energy consumption and carbon emissions. Sustainable practices in graphite mining and recycling contribute to reducing ecological footprints, whereas diamond production demands ongoing innovations to minimize environmental harm and improve lifecycle sustainability.
Market Value and Economic Importance
Graphite carbon commands a significant market value due to its widespread use in industries such as lubricants, batteries, and steelmaking, driven by demand for electric vehicles and renewable energy storage solutions. Diamond carbon, while rarer and more valuable per carat in the gemstone market, also holds critical economic importance in cutting, grinding, and drilling applications because of its unparalleled hardness. The economic impact of both forms is shaped by their distinct industrial applications, with graphite fueling technological advancements and diamond supporting high-precision manufacturing and luxury sectors.
Graphite carbon vs Diamond carbon Infographic
 materialdif.com
materialdif.com