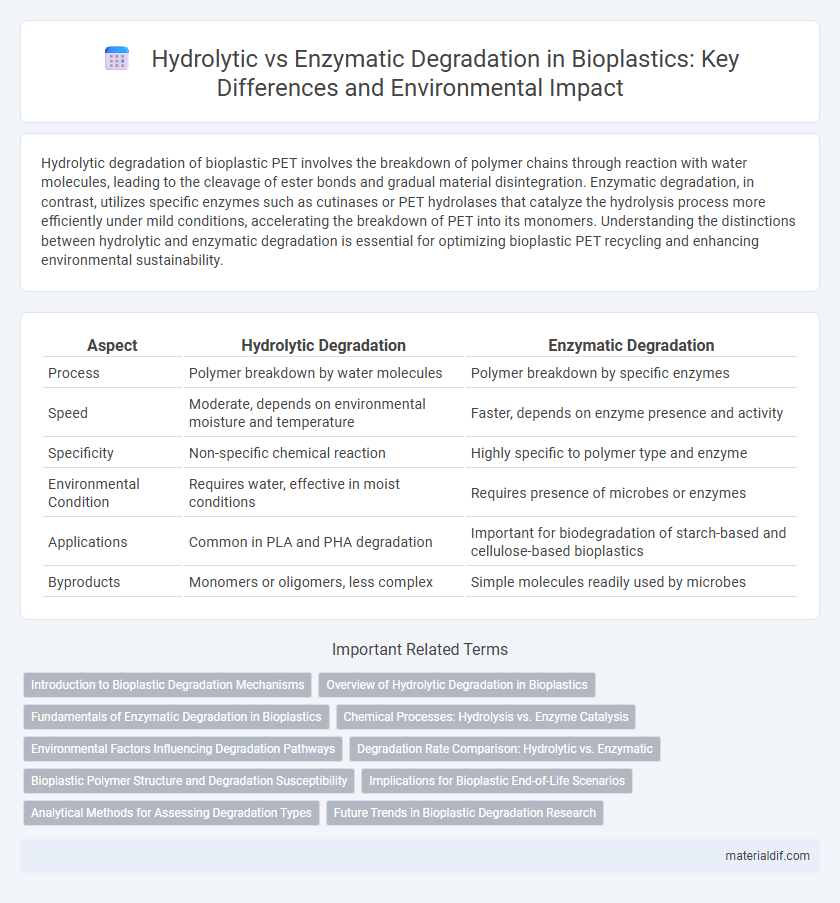
Hydrolytic degradation of bioplastic PET involves the breakdown of polymer chains through reaction with water molecules, leading to the cleavage of ester bonds and gradual material disintegration. Enzymatic degradation, in contrast, utilizes specific enzymes such as cutinases or PET hydrolases that catalyze the hydrolysis process more efficiently under mild conditions, accelerating the breakdown of PET into its monomers. Understanding the distinctions between hydrolytic and enzymatic degradation is essential for optimizing bioplastic PET recycling and enhancing environmental sustainability.
Table of Comparison
| Aspect | Hydrolytic Degradation | Enzymatic Degradation |
|---|---|---|
| Process | Polymer breakdown by water molecules | Polymer breakdown by specific enzymes |
| Speed | Moderate, depends on environmental moisture and temperature | Faster, depends on enzyme presence and activity |
| Specificity | Non-specific chemical reaction | Highly specific to polymer type and enzyme |
| Environmental Condition | Requires water, effective in moist conditions | Requires presence of microbes or enzymes |
| Applications | Common in PLA and PHA degradation | Important for biodegradation of starch-based and cellulose-based bioplastics |
| Byproducts | Monomers or oligomers, less complex | Simple molecules readily used by microbes |
Introduction to Bioplastic Degradation Mechanisms
Bioplastic degradation involves hydrolytic and enzymatic processes that break down polymers into smaller molecules. Hydrolytic degradation occurs through water-induced cleavage of polymer chains, while enzymatic degradation relies on specific enzymes to catalyze polymer breakdown. Understanding these mechanisms is essential for optimizing bioplastic design and environmental impact.
Overview of Hydrolytic Degradation in Bioplastics
Hydrolytic degradation in bioplastics involves the cleavage of polymer chains through reaction with water molecules, leading to the breakdown of ester bonds commonly found in polylactic acid (PLA) and polyhydroxyalkanoates (PHA). This process is significantly influenced by environmental factors such as temperature, pH, and moisture levels, with increased hydrolysis rates observed under elevated temperatures and humid conditions. Compared to enzymatic degradation, hydrolytic degradation is generally a slower, non-biological mechanism that initiates the polymer fragmentation before microbes can further metabolize the smaller molecules.
Fundamentals of Enzymatic Degradation in Bioplastics
Enzymatic degradation in bioplastics relies on specific enzymes that catalyze the breakdown of polymer chains into smaller molecules such as monomers or oligomers, facilitating microbial assimilation and complete biodegradation. This process is influenced by factors like enzyme type, substrate specificity, temperature, pH, and the polymer's molecular structure, including crystallinity and hydrophilicity. Unlike hydrolytic degradation, enzymatic degradation offers a targeted and efficient mechanism for depolymerization, critical for designing sustainable bioplastics with controlled degradation rates.
Chemical Processes: Hydrolysis vs. Enzyme Catalysis
Hydrolytic degradation involves the chemical breakdown of bioplastics through the reaction with water molecules, resulting in the cleavage of polymer chains via hydrolysis. Enzymatic degradation relies on specific enzymes that catalyze the breakdown of bioplastics by targeting particular bonds within the polymer structure, often leading to faster and more selective decomposition. The chemical process of hydrolysis is a non-biological mechanism driven by water concentration and environmental conditions, whereas enzyme catalysis depends on biological agents that enhance reaction rates under milder conditions.
Environmental Factors Influencing Degradation Pathways
Hydrolytic degradation of bioplastics is primarily influenced by moisture, temperature, and pH levels, which accelerate the cleavage of polymer bonds through water interaction. Enzymatic degradation depends heavily on the presence and activity of specific microorganisms, as well as environmental parameters such as nutrient availability, temperature, and oxygen levels that regulate enzyme production. Variations in soil composition, humidity, and microbial diversity critically determine the dominant degradation pathway and the overall breakdown rate of bioplastic materials.
Degradation Rate Comparison: Hydrolytic vs. Enzymatic
Hydrolytic degradation of bioplastics involves the cleavage of polymer chains by water molecules, typically resulting in a slower degradation rate compared to enzymatic degradation, which is accelerated by specific enzymes targeting polymer bonds. Enzymatic degradation exhibits higher efficiency due to enzyme-substrate specificity, often leading to rapid breakdown of polyesters like PLA and PHA under controlled environmental conditions. The degradation rate of enzymatic pathways can be up to several times faster than hydrolytic mechanisms, making enzymatic degradation more favorable for applications requiring quicker biodegradability.
Bioplastic Polymer Structure and Degradation Susceptibility
Hydrolytic degradation of bioplastic primarily targets polymer chains with hydrolyzable ester bonds, leading to chain scission through water molecule interaction, which is highly influenced by the polymer's crystallinity and hydrophilicity. Enzymatic degradation depends on specific enzymes recognizing and breaking down polymer backbones, often requiring amorphous regions and accessible ester or ether linkages within the bioplastic structure for effective biodegradation. Polymers like polylactic acid (PLA) and polyhydroxyalkanoates (PHA) exhibit varying susceptibility due to differences in their molecular weight, crystallinity, and functional group accessibility, determining their degradation rate under hydrolytic or enzymatic conditions.
Implications for Bioplastic End-of-Life Scenarios
Hydrolytic degradation of bioplastics involves the cleavage of polymer chains by water molecules, resulting in gradual material breakdown under controlled moisture and temperature conditions. Enzymatic degradation utilizes specific enzymes to catalyze the decomposition of bioplastic polymers, often leading to faster and more complete mineralization in natural environments like soil and compost. Understanding the distinct mechanisms and rates of hydrolytic versus enzymatic degradation is crucial for optimizing bioplastic end-of-life strategies, including compostability, recyclability, and environmental impact reduction.
Analytical Methods for Assessing Degradation Types
Analytical methods for assessing hydrolytic degradation of bioplastics often involve measuring changes in molecular weight using gel permeation chromatography (GPC) and monitoring weight loss in controlled aqueous environments. Enzymatic degradation is typically evaluated through enzyme assays that quantify specific enzymatic activity and scanning electron microscopy (SEM) to observe surface morphology changes. Spectroscopic techniques like Fourier-transform infrared spectroscopy (FTIR) provide molecular-level insights distinguishing hydrolytic bond cleavage from enzyme-catalyzed reactions.
Future Trends in Bioplastic Degradation Research
Hydrolytic degradation and enzymatic degradation represent two pivotal mechanisms driving bioplastic breakdown, with hydrolysis relying on water-mediated cleavage and enzymatic degradation involving biocatalysts that target polymer chains. Future trends emphasize integrating advanced enzyme engineering and synthetic biology to enhance enzymatic specificity and efficiency, facilitating faster and more controlled bioplastic decomposition. Emerging research prioritizes developing hybrid degradation systems that combine hydrolytic and enzymatic processes to optimize environmental sustainability and waste management.
Hydrolytic degradation vs Enzymatic degradation Infographic
 materialdif.com
materialdif.com