Ferrous alloys contain iron as their primary element, providing high strength, durability, and magnetic properties, making them ideal for construction and automotive applications. Non-ferrous alloys, which exclude iron, offer superior resistance to corrosion, lightweight characteristics, and excellent conductivity, frequently used in aerospace, electrical, and marine industries. Understanding the distinct properties of ferrous versus non-ferrous alloys is crucial for selecting the right material based on mechanical requirements and environmental conditions.
Table of Comparison
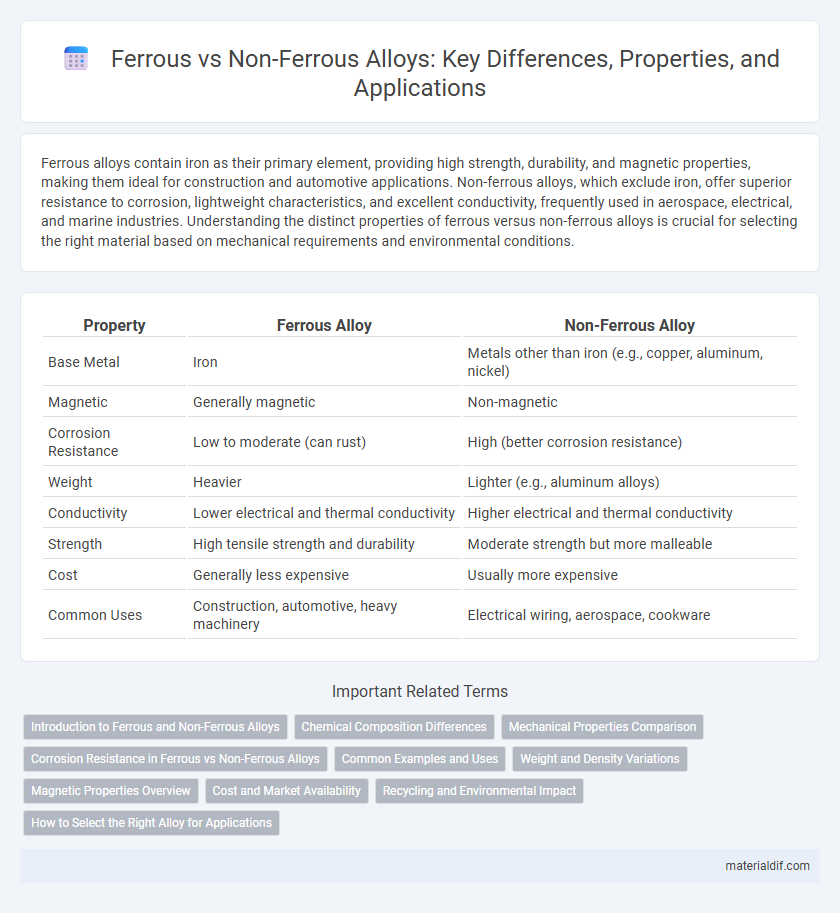
| Property | Ferrous Alloy | Non-Ferrous Alloy |
|---|---|---|
| Base Metal | Iron | Metals other than iron (e.g., copper, aluminum, nickel) |
| Magnetic | Generally magnetic | Non-magnetic |
| Corrosion Resistance | Low to moderate (can rust) | High (better corrosion resistance) |
| Weight | Heavier | Lighter (e.g., aluminum alloys) |
| Conductivity | Lower electrical and thermal conductivity | Higher electrical and thermal conductivity |
| Strength | High tensile strength and durability | Moderate strength but more malleable |
| Cost | Generally less expensive | Usually more expensive |
| Common Uses | Construction, automotive, heavy machinery | Electrical wiring, aerospace, cookware |
Introduction to Ferrous and Non-Ferrous Alloys
Ferrous alloys primarily contain iron as their base metal, offering high tensile strength and magnetic properties, making them essential in construction and automotive industries. Non-ferrous alloys lack iron, providing superior resistance to corrosion, lightweight characteristics, and excellent electrical conductivity, widely used in aerospace and electrical applications. Understanding the distinct composition and properties of ferrous versus non-ferrous alloys helps optimize material selection based on specific performance requirements.
Chemical Composition Differences
Ferrous alloys primarily consist of iron as the base element, combined with carbon and other elements like manganese, chromium, and nickel to enhance strength and corrosion resistance. Non-ferrous alloys lack iron and are mainly composed of metals such as aluminum, copper, zinc, and magnesium, offering superior resistance to rust and lighter weight. The distinct chemical compositions define their applications, with ferrous alloys used in construction and machinery, while non-ferrous alloys serve in aerospace, electronics, and corrosion-prone environments.
Mechanical Properties Comparison
Ferrous alloys, primarily composed of iron, exhibit superior tensile strength and hardness, making them ideal for heavy-duty applications, while non-ferrous alloys offer better corrosion resistance and lighter weight, enhancing their suitability for aerospace and marine environments. The mechanical properties of ferrous alloys include high impact resistance and wear resistance due to the presence of carbon and other strengthening elements. Non-ferrous alloys, such as aluminum and copper-based alloys, provide excellent ductility and thermal conductivity, which are critical for electrical and heat transfer applications.
Corrosion Resistance in Ferrous vs Non-Ferrous Alloys
Ferrous alloys, primarily composed of iron, generally have lower corrosion resistance compared to non-ferrous alloys, which include metals like aluminum, copper, and nickel known for their inherent resistance to oxidation and rust. Non-ferrous alloys resist corrosion due to their stable oxide layers and absence of iron, reducing susceptibility to rust and environmental degradation. In applications demanding high corrosion resistance, non-ferrous alloys are preferred, especially in marine, chemical, and outdoor environments where exposure to moisture and corrosive agents is frequent.
Common Examples and Uses
Ferrous alloys primarily consist of iron and include common examples like carbon steel, stainless steel, and cast iron, widely used in construction, automotive manufacturing, and infrastructure due to their high strength and magnetic properties. Non-ferrous alloys, such as aluminum alloys, copper alloys (like bronze and brass), and titanium alloys, are valued for their corrosion resistance, lightweight characteristics, and electrical conductivity, making them ideal for aerospace, electrical wiring, and marine applications. The choice between ferrous and non-ferrous alloys depends on required mechanical properties, environmental resistance, and specific use-case demands.
Weight and Density Variations
Ferrous alloys, primarily composed of iron, exhibit higher density values typically around 7.8 g/cm3, contributing to their heavier weight compared to non-ferrous alloys. Non-ferrous alloys, such as aluminum and copper-based materials, have lower densities ranging from 2.7 g/cm3 to 8.9 g/cm3, resulting in lighter and more corrosion-resistant applications. Weight and density variations between ferrous and non-ferrous alloys significantly influence their selection criteria in automotive, aerospace, and construction industries.
Magnetic Properties Overview
Ferrous alloys contain iron, giving them strong magnetic properties, which makes them highly suitable for applications requiring magnetism, such as transformers and electric motors. Non-ferrous alloys, lacking significant iron content, generally exhibit weak or no magnetism, resulting in their use where magnetic interference must be minimized, like in aerospace and electronic components. Understanding the magnetic behavior differences between ferrous and non-ferrous alloys is crucial for selecting materials in electromagnetic and structural engineering.
Cost and Market Availability
Ferrous alloys, primarily composed of iron, tend to be more cost-effective due to their abundant raw materials and established manufacturing processes, making them widely available in global markets. Non-ferrous alloys, such as aluminum, copper, and titanium, generally incur higher costs due to more complex extraction and refining methods, limiting their market availability and increasing price volatility. Market demand for ferrous alloys is driven by construction and automotive industries, whereas non-ferrous alloys are preferred in aerospace and electronics for their specialized properties despite higher costs and limited supply.
Recycling and Environmental Impact
Ferrous alloys, primarily composed of iron, are highly recyclable with steel recycling significantly reducing energy consumption and carbon emissions compared to primary production. Non-ferrous alloys, such as aluminum and copper, also offer excellent recyclability, but their recycling processes often involve higher energy inputs due to complex extraction methods. Both alloy types contribute to resource conservation and environmental sustainability, though ferrous alloys generally have a lower overall environmental impact when recycled efficiently.
How to Select the Right Alloy for Applications
Selecting the right alloy for applications requires understanding the specific properties needed, such as strength, corrosion resistance, and conductivity. Ferrous alloys, primarily composed of iron, offer high tensile strength and durability, making them ideal for structural and heavy machinery applications. Non-ferrous alloys, containing metals like aluminum, copper, and nickel, provide superior corrosion resistance and electrical conductivity, suitable for aerospace, electrical components, and marine environments.
Ferrous alloy vs Non-ferrous alloy Infographic
 materialdif.com
materialdif.com