Zinc anodes offer superior corrosion resistance in saltwater environments compared to magnesium anodes, making them ideal for marine applications on boats and ships. Magnesium anodes provide higher protection efficiency in freshwater but tend to corrode faster in saltwater, requiring more frequent replacement. Choosing between zinc and magnesium anodes depends on the specific water conditions and maintenance preferences to ensure optimal cathodic protection.
Table of Comparison
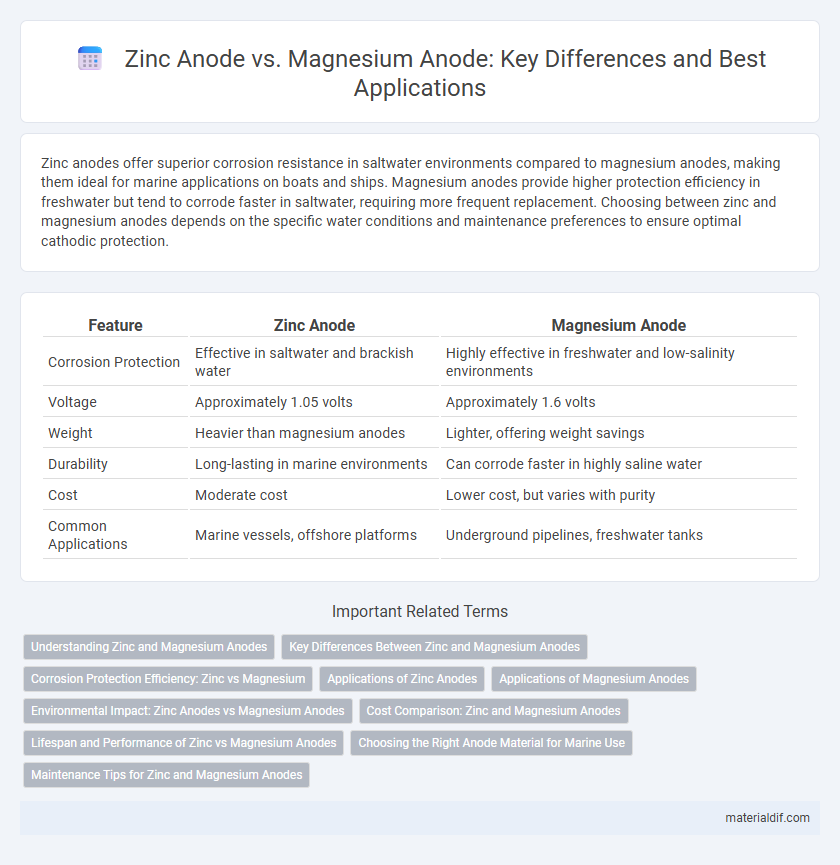
| Feature | Zinc Anode | Magnesium Anode |
|---|---|---|
| Corrosion Protection | Effective in saltwater and brackish water | Highly effective in freshwater and low-salinity environments |
| Voltage | Approximately 1.05 volts | Approximately 1.6 volts |
| Weight | Heavier than magnesium anodes | Lighter, offering weight savings |
| Durability | Long-lasting in marine environments | Can corrode faster in highly saline water |
| Cost | Moderate cost | Lower cost, but varies with purity |
| Common Applications | Marine vessels, offshore platforms | Underground pipelines, freshwater tanks |
Understanding Zinc and Magnesium Anodes
Zinc anodes offer excellent corrosion protection for steel structures in marine environments by providing a reliable sacrificial layer, while magnesium anodes provide a stronger driving voltage ideal for freshwater applications with higher resistivity. The electrochemical properties of zinc make it stable and long-lasting in saltwater, whereas magnesium, with its higher electrochemical potential, corrodes faster but delivers more aggressive protection in less conductive waters. Understanding the specific environmental conditions and metal compatibility is crucial when selecting between zinc and magnesium anodes for effective cathodic protection.
Key Differences Between Zinc and Magnesium Anodes
Zinc anodes offer slower corrosion rates and longer service life compared to magnesium anodes, making them ideal for marine environments with moderate salinity. Magnesium anodes provide higher electrochemical driving voltage, making them more effective in low conductivity soils or freshwater applications where aggressive corrosion protection is required. The choice between zinc and magnesium anodes hinges on environmental conditions, conductivity, and required lifespan for cathodic protection systems.
Corrosion Protection Efficiency: Zinc vs Magnesium
Zinc anodes provide reliable corrosion protection with moderate electrochemical activity, making them effective in marine environments but slower to degrade compared to magnesium. Magnesium anodes exhibit higher electrochemical potential, resulting in faster corrosion protection and more aggressive sacrificial action, ideal for freshwater applications where rapid metal protection is critical. The choice between zinc and magnesium anodes depends on specific environmental conditions, with zinc preferred for saltwater and magnesium for freshwater due to their differing corrosion protection efficiencies.
Applications of Zinc Anodes
Zinc anodes are widely used in marine and industrial applications due to their excellent corrosion protection in seawater and brackish water environments, providing sacrificial protection for steel structures such as ship hulls, offshore platforms, and pipelines. Their lower driving voltage compared to magnesium anodes makes zinc anodes ideal for environments with moderate salinity where controlled protection is essential to prevent overprotection and hydrogen embrittlement. Zinc anodes also find effective use in potable water systems and cooling water applications where their stability and predictable consumption rate ensure long-lasting corrosion protection.
Applications of Magnesium Anodes
Magnesium anodes are primarily utilized in environments where high corrosion rates demand a more reactive sacrificial material, such as in soil and freshwater corrosion protection for underground pipelines, tanks, and cathodic protection systems. Their higher driving voltage compared to zinc anodes makes them ideal for protecting steel structures in aggressive environments, ensuring enhanced durability and prevention of metal loss. Magnesium anodes demonstrate superior performance in applications with low resistivity soils or waters, making them a preferred choice for oil and gas pipelines, water heaters, and marine vessels.
Environmental Impact: Zinc Anodes vs Magnesium Anodes
Zinc anodes produce fewer harmful byproducts and pose less risk of heavy metal contamination in marine environments compared to magnesium anodes, which can accelerate corrosion in certain alloys and introduce higher alkalinity levels to water. Magnesium anodes, while offering higher driving voltage for cathodic protection, tend to dissolve more rapidly, resulting in increased metal ion discharge that can disrupt local ecosystems. The environmental footprint of zinc anodes is generally considered lower, making them a preferred choice for long-term applications in sensitive aquatic habitats.
Cost Comparison: Zinc and Magnesium Anodes
Zinc anodes generally cost more per pound than magnesium anodes, but their lower consumption rate often results in a longer lifespan, making them cost-effective over time. Magnesium anodes are less expensive upfront and provide higher driving voltage, which is beneficial in low-resistivity environments such as freshwater. The choice between zinc and magnesium anodes depends on balancing initial cost with environmental factors and expected maintenance intervals.
Lifespan and Performance of Zinc vs Magnesium Anodes
Zinc anodes offer a longer lifespan and better corrosion resistance in saltwater environments compared to magnesium anodes, which tend to degrade faster. Magnesium anodes provide higher driving voltage, making them more effective in freshwater applications but with a shorter operational life. The choice between zinc and magnesium anodes hinges on balancing durability and performance based on water salinity and environmental conditions.
Choosing the Right Anode Material for Marine Use
Zinc anodes offer reliable corrosion protection in saltwater environments due to their moderate electrochemical potential and cost-effectiveness, making them a common choice for marine applications. Magnesium anodes provide higher electrochemical activity, making them more suitable for freshwater environments where increased protection is necessary, but they degrade faster in marine saltwater conditions. Selecting the right anode material depends on water type, temperature, salinity, and vessel design to ensure optimum sacrificial corrosion protection.
Maintenance Tips for Zinc and Magnesium Anodes
Zinc anodes require regular inspection to ensure effective corrosion protection, with replacement typically needed every 1 to 3 years depending on water salinity and usage. Magnesium anodes, while offering higher driving voltage and better protection in freshwater, demand more frequent checks and replacements due to their faster consumption rate. Both anode types benefit from keeping the mounting surface clean and free of marine growth to maintain optimal electrical contact and prolong service life.
Zinc Anode vs Magnesium Anode Infographic
 materialdif.com
materialdif.com