Sherardizing and Parkerizing are two metal coating processes used to enhance the corrosion resistance and durability of zinc-plated steel. Sherardizing involves a dry diffusion method where zinc powder is heated with the metal, creating a uniform, abrasive-resistant zinc-iron alloy layer. Parkerizing, on the other hand, is a wet chemical phosphating treatment that produces a porous, matte finish primarily used for improved paint adhesion and moderate corrosion protection.
Table of Comparison
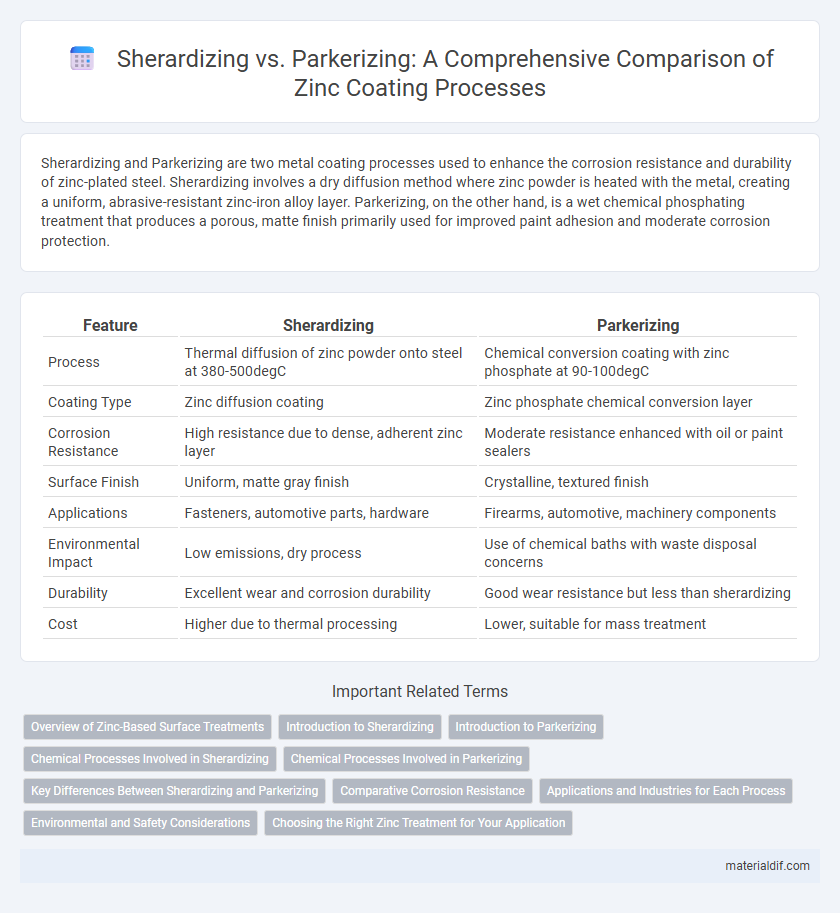
| Feature | Sherardizing | Parkerizing |
|---|---|---|
| Process | Thermal diffusion of zinc powder onto steel at 380-500degC | Chemical conversion coating with zinc phosphate at 90-100degC |
| Coating Type | Zinc diffusion coating | Zinc phosphate chemical conversion layer |
| Corrosion Resistance | High resistance due to dense, adherent zinc layer | Moderate resistance enhanced with oil or paint sealers |
| Surface Finish | Uniform, matte gray finish | Crystalline, textured finish |
| Applications | Fasteners, automotive parts, hardware | Firearms, automotive, machinery components |
| Environmental Impact | Low emissions, dry process | Use of chemical baths with waste disposal concerns |
| Durability | Excellent wear and corrosion durability | Good wear resistance but less than sherardizing |
| Cost | Higher due to thermal processing | Lower, suitable for mass treatment |
Overview of Zinc-Based Surface Treatments
Sherardizing and Parkerizing are zinc-based surface treatments that enhance metal corrosion resistance through distinct processes. Sherardizing involves diffusing zinc vapor into steel at high temperatures, creating a robust, uniform zinc-iron alloy coating ideal for automotive and hardware applications. Parkerizing forms a crystalline phosphate coating with zinc salts, offering excellent corrosion protection and paint adhesion primarily for firearms and military equipment.
Introduction to Sherardizing
Sherardizing is a thermal diffusion zinc coating process that provides excellent corrosion resistance by embedding zinc into the surface of steel components through controlled heating and agitation. Unlike Parkerizing, which deposits a phosphate conversion coating, Sherardizing creates a metallurgical bond, resulting in a uniform, abrasion-resistant zinc layer ideal for small or complex parts. This method enhances durability and prolongs the lifespan of steel by forming a robust zinc-iron alloy, making it a preferred choice in automotive and industrial applications.
Introduction to Parkerizing
Parkerizing is a metal treatment process that applies a protective phosphate coating, primarily used on steel surfaces to enhance corrosion resistance and paint adhesion. Unlike sherardizing, which involves a zinc vapor diffusion method, Parkerizing uses a chemical bath containing phosphoric acid and metal phosphates to produce a durable, matte finish. This process is widely applied in firearm manufacturing and automotive industries to improve surface durability and prevent rust.
Chemical Processes Involved in Sherardizing
Sherardizing is a chemical diffusion process where steel parts are heated in a closed rotating drum containing zinc powder, promoting zinc vapor to diffuse onto the substrate, forming a metallurgical bond. Unlike Parkerizing, which relies on a phosphoric acid solution to create a phosphate conversion coating, Sherardizing uses high-temperature zinc vapor to produce a uniform, corrosion-resistant zinc layer. This process enhances adhesion and corrosion protection by creating a zinc-iron alloy layer through solid-state diffusion rather than a surface phosphate film.
Chemical Processes Involved in Parkerizing
Parkerizing, also known as phosphating, involves a chemical reaction where metal surfaces, typically steel or iron, are immersed in a solution containing phosphoric acid and metal ions such as zinc or manganese, forming a layer of insoluble metal phosphate crystals. This conversion coating enhances corrosion resistance and provides a good base for paint adhesion by creating a porous surface. Unlike sherardizing, which relies on zinc vapor diffusion, parkerizing employs a direct chemical interaction to produce a durable phosphate film on the substrate.
Key Differences Between Sherardizing and Parkerizing
Sherardizing involves a dry heat diffusion process where zinc vapor bonds with the metal surface, creating a highly corrosion-resistant coating, while Parkerizing uses a chemical phosphate treatment forming a porous layer that enhances paint adhesion and corrosion resistance. Sherardizing provides uniform coating on complex geometries, typically used for small steel parts, whereas Parkerizing is favored for larger surfaces and firearm components due to its matte finish and rust-preventive qualities. The zinc coating thickness in Sherardizing usually ranges from 5 to 25 microns, contrasting with Parkerizing's thinner phosphate layers that depend on process duration and solution concentration.
Comparative Corrosion Resistance
Sherardizing offers superior corrosion resistance compared to Parkerizing due to its diffusion-based zinc coating that provides a uniform, tightly bonded layer on steel surfaces. Parkerizing forms a phosphate layer that offers moderate corrosion protection but is more prone to wear and less effective in harsh environments. Sherardized coatings typically exhibit enhanced durability, especially in salt spray tests, making them preferable for long-term corrosion prevention.
Applications and Industries for Each Process
Sherardizing enhances corrosion resistance by diffusing zinc into steel surfaces, making it ideal for automotive parts, fasteners, and hardware exposed to harsh environments. Parkerizing creates a phosphate conversion coating primarily used in firearms, military equipment, and industrial tools to improve paint adhesion and wear resistance. Both processes serve industries requiring durable metal finishes, but Sherardizing is preferred for structural components, while Parkerizing suits protective coatings in tactical and mechanical applications.
Environmental and Safety Considerations
Sherardizing involves a closed-vessel process that produces minimal hazardous waste and has lower emissions compared to Parkerizing, which uses acidic phosphate solutions generating more chemical runoff and sludge disposal challenges. Sherardizing employs zinc dust and heat, reducing the risk of handling corrosive chemicals, while Parkerizing requires strict management of phosphoric acid and heavy metals for worker safety and environmental compliance. The closed nature of Sherardizing makes it a safer and more environmentally friendly option for zinc coating in industrial applications.
Choosing the Right Zinc Treatment for Your Application
Sherardizing offers superior corrosion resistance by diffusively bonding zinc into the metal surface, making it ideal for complex shapes and high-stress applications. Parkerizing, also known as phosphating, provides a thinner, matte zinc phosphate coating that improves paint adhesion and is best suited for firearm parts and lighter-duty corrosion protection. Selecting the right zinc treatment depends on factors like component geometry, environmental exposure, and required durability, with sherardizing preferred for heavy-duty protection and parkerizing favored for surface preparation before painting.
Sherardizing vs Parkerizing Infographic
 materialdif.com
materialdif.com