Passivation enhances zinc pet coatings by forming a thin, protective oxide layer that improves corrosion resistance without altering the surface appearance. Anodizing, by contrast, involves an electrochemical process that thickens the oxide layer, significantly boosting durability and wear resistance while adding a decorative finish. Choosing between passivation and anodizing depends on the desired balance of aesthetic appeal, corrosion protection, and mechanical strength for zinc pet applications.
Table of Comparison
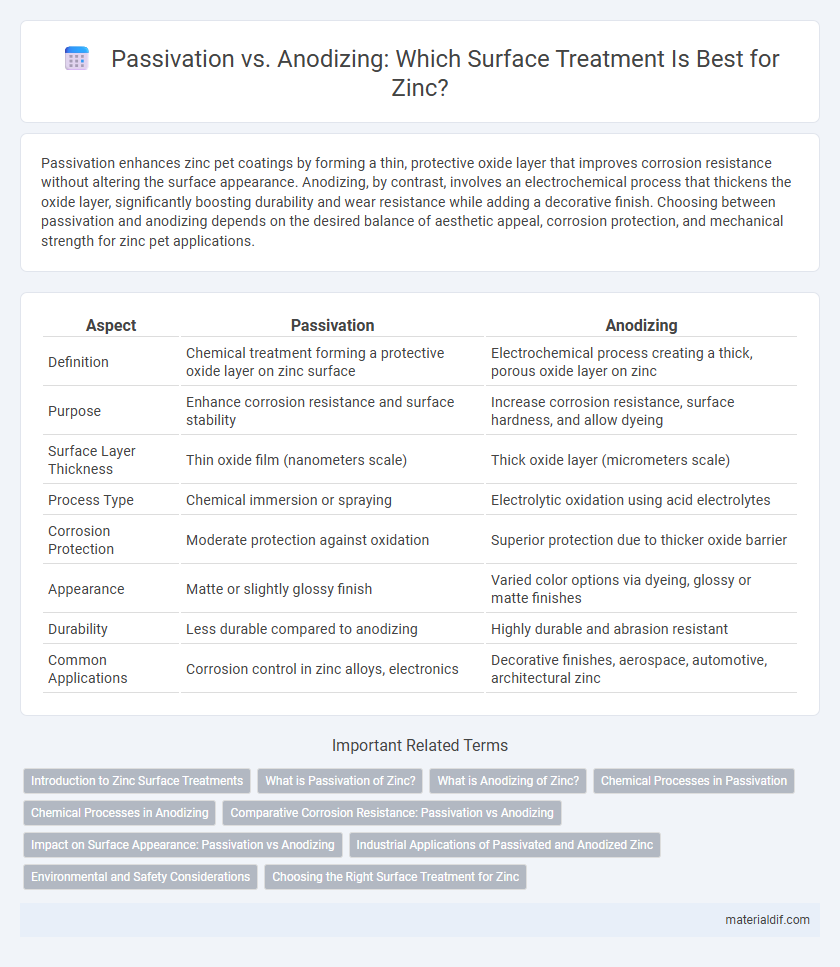
| Aspect | Passivation | Anodizing |
|---|---|---|
| Definition | Chemical treatment forming a protective oxide layer on zinc surface | Electrochemical process creating a thick, porous oxide layer on zinc |
| Purpose | Enhance corrosion resistance and surface stability | Increase corrosion resistance, surface hardness, and allow dyeing |
| Surface Layer Thickness | Thin oxide film (nanometers scale) | Thick oxide layer (micrometers scale) |
| Process Type | Chemical immersion or spraying | Electrolytic oxidation using acid electrolytes |
| Corrosion Protection | Moderate protection against oxidation | Superior protection due to thicker oxide barrier |
| Appearance | Matte or slightly glossy finish | Varied color options via dyeing, glossy or matte finishes |
| Durability | Less durable compared to anodizing | Highly durable and abrasion resistant |
| Common Applications | Corrosion control in zinc alloys, electronics | Decorative finishes, aerospace, automotive, architectural zinc |
Introduction to Zinc Surface Treatments
Zinc surface treatments primarily include passivation and anodizing, which enhance corrosion resistance and durability. Passivation involves creating a thin, protective oxide layer on the zinc surface, reducing oxidation and improving adhesion for subsequent coatings. Anodizing, on the other hand, thickens the natural oxide layer through an electrochemical process, offering superior protection and a more decorative finish.
What is Passivation of Zinc?
Passivation of zinc involves creating a protective, thin oxide layer on the metal surface to prevent corrosion and enhance durability. This chemical treatment improves zinc's resistance to environmental factors by reducing its reactivity with air and moisture. Unlike anodizing, which thickens the oxide layer through electrochemical processes, passivation relies on chemical or immersion methods to stabilize the zinc surface.
What is Anodizing of Zinc?
Anodizing of zinc is an electrochemical process that enhances corrosion resistance by forming a controlled oxide layer on the metal surface. This oxide layer improves zinc's durability, adhesion, and aesthetic appearance, making it ideal for industrial and architectural applications. Unlike passivation, anodizing actively modifies the surface through anodic oxidation rather than creating a passive film via chemical treatment.
Chemical Processes in Passivation
Passivation of zinc involves chemical treatment with chromates or phosphates to form a thin, protective oxide layer that inhibits corrosion by enhancing the metal's natural resistance. This process modifies the zinc surface at a molecular level, preventing oxidation and prolonging durability without altering the metal's bulk properties. Unlike anodizing, which uses electrolytic oxidation to thicken the oxide layer, passivation relies on chemical conversion coatings for surface protection.
Chemical Processes in Anodizing
Anodizing zinc involves electrochemical oxidation, where the zinc surface acts as an anode in an electrolytic cell, forming a dense, controlled oxide layer that enhances corrosion resistance and surface hardness. This chemical process differs from passivation, which relies on applying chemical inhibitors or treatments to form a thin, passive film without significant alteration of the metal's surface structure. Anodized zinc layers provide superior durability and adhesion properties compared to passivated coatings due to their porous and tightly bound oxide composition.
Comparative Corrosion Resistance: Passivation vs Anodizing
Passivation creates a thin, inert oxide layer on zinc surfaces that inhibits oxidation and enhances corrosion resistance primarily in mild environments. Anodizing, however, produces a thicker, more durable oxide coating that offers superior corrosion protection, especially under harsh conditions or prolonged exposure to moisture and chemicals. Comparative studies demonstrate anodized zinc's increased resistance to pitting and environmental degradation compared to passivated zinc.
Impact on Surface Appearance: Passivation vs Anodizing
Passivation enhances zinc's corrosion resistance by forming a thin, clear oxide layer that preserves the metal's natural luster and prevents tarnishing. Anodizing creates a thicker, colored oxide film on zinc surfaces, offering customizable aesthetic finishes but resulting in a more matte, less reflective appearance. Comparing surface appearance, passivation maintains zinc's original shine, while anodizing decisively alters its visual texture and color options.
Industrial Applications of Passivated and Anodized Zinc
Passivated zinc is widely used in industrial applications requiring enhanced corrosion resistance, such as automotive parts, fasteners, and electrical components, due to its effective barrier against oxidation. Anodized zinc, though less common than anodized aluminum, is applied in industries demanding decorative finishes and improved surface hardness, including consumer electronics and architectural elements. The choice between passivation and anodizing depends on the specific environmental exposure and mechanical performance requirements of the zinc-coated products.
Environmental and Safety Considerations
Passivation of zinc enhances its corrosion resistance by forming a non-toxic, thin oxide layer, using environmentally friendly chemicals like citric acid, minimizing hazardous waste. Anodizing produces a thicker oxide coating but often involves acidic electrolytes such as sulfuric acid, generating more hazardous effluents requiring careful disposal. Considering environmental impact and worker safety, passivation offers a safer alternative with reduced chemical hazards and lower ecological footprint compared to anodizing.
Choosing the Right Surface Treatment for Zinc
Passivation creates a thin, protective oxide layer on zinc that enhances corrosion resistance without altering the metal's appearance, ideal for indoor or mild environments. Anodizing thickens the oxide coating through electrochemical processes, providing superior durability and color options, suitable for harsher, outdoor conditions. Selecting between passivation and anodizing depends on application-specific factors such as exposure, aesthetic requirements, and longevity demands of the zinc components.
Passivation vs Anodizing Infographic
 materialdif.com
materialdif.com