Tin oxide and tin sulfide are both valuable compounds with distinct properties and applications. Tin oxide is commonly used as a transparent conducting layer in electronic devices due to its high conductivity and transparency, while tin sulfide is favored in photovoltaics for its suitable band gap and earth-abundant, non-toxic elements. Understanding their differences in electrical and optical characteristics is crucial for optimizing performance in sensors, solar cells, and optoelectronic devices.
Table of Comparison
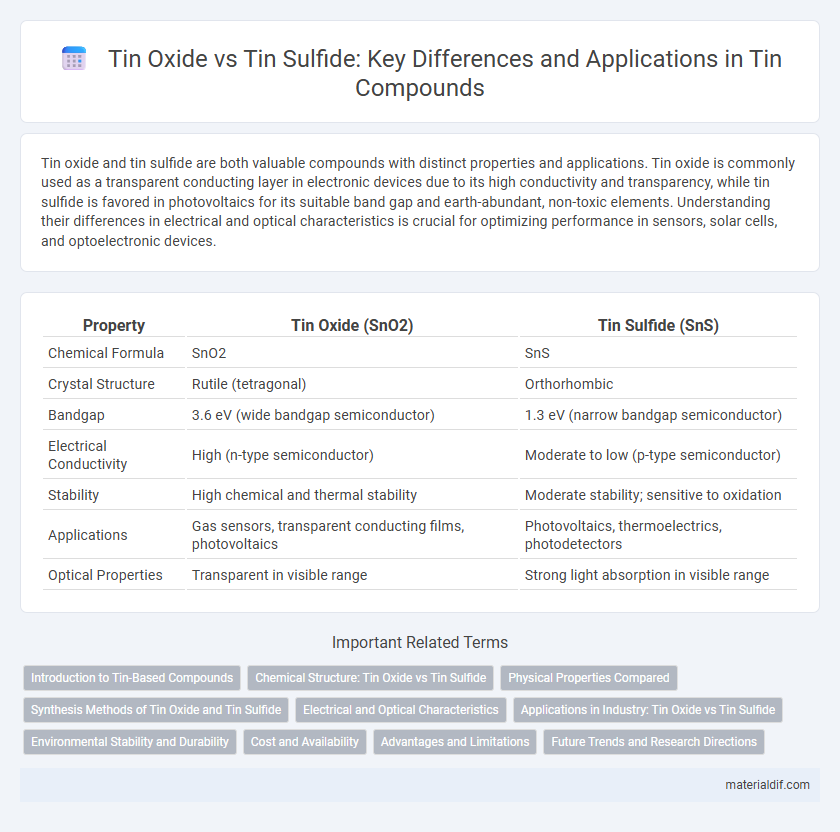
| Property | Tin Oxide (SnO2) | Tin Sulfide (SnS) |
|---|---|---|
| Chemical Formula | SnO2 | SnS |
| Crystal Structure | Rutile (tetragonal) | Orthorhombic |
| Bandgap | 3.6 eV (wide bandgap semiconductor) | 1.3 eV (narrow bandgap semiconductor) |
| Electrical Conductivity | High (n-type semiconductor) | Moderate to low (p-type semiconductor) |
| Stability | High chemical and thermal stability | Moderate stability; sensitive to oxidation |
| Applications | Gas sensors, transparent conducting films, photovoltaics | Photovoltaics, thermoelectrics, photodetectors |
| Optical Properties | Transparent in visible range | Strong light absorption in visible range |
Introduction to Tin-Based Compounds
Tin oxide (SnO2) and tin sulfide (SnS) are prominent tin-based compounds with distinct chemical and physical properties influencing their applications. Tin oxide is widely utilized in gas sensors, transparent conducting films, and catalysis due to its high electrical conductivity and optical transparency. Tin sulfide exhibits promising semiconductor behavior with potential in photovoltaic devices and photodetectors thanks to its suitable bandgap and earth-abundant, non-toxic nature.
Chemical Structure: Tin Oxide vs Tin Sulfide
Tin oxide (SnO2) features a tetragonal rutile crystal structure where each tin atom is coordinated by six oxygen atoms, creating a robust network that imparts high stability and wide bandgap semiconductor properties. Tin sulfide (SnS), in contrast, adopts an orthorhombic layered structure with tin atoms bonded to sulfur atoms, resulting in anisotropic electrical and optical characteristics suitable for photovoltaic applications. The differing atomic arrangements and bonding types in tin oxide and tin sulfide lead to distinct electronic structures, influencing their respective conductivity and bandgap energies.
Physical Properties Compared
Tin oxide (SnO2) exhibits high hardness, a wide bandgap of approximately 3.6 eV, and strong optical transparency, making it ideal for applications in transparent conducting films. In contrast, tin sulfide (SnS) has a narrower bandgap around 1.3 eV, a softer crystalline structure, and lower optical transparency, favoring its use in photovoltaic and semiconductor devices. The distinct differences in thermal conductivity, electrical resistivity, and structural stability between SnO2 and SnS significantly influence their suitability in various electronic and optoelectronic applications.
Synthesis Methods of Tin Oxide and Tin Sulfide
Tin oxide synthesis commonly employs sol-gel processes, hydrothermal methods, and chemical vapor deposition to achieve high-purity nanoparticles with controlled morphologies. Tin sulfide is often synthesized via solvothermal techniques, chemical bath deposition, and sulfurization of tin precursors, facilitating the formation of various crystal phases like SnS, SnS2, and Sn2S3. These methods allow precise control over particle size, stoichiometry, and structural properties crucial for optimizing electronic and optoelectronic applications.
Electrical and Optical Characteristics
Tin oxide (SnO2) exhibits high electrical conductivity and transparency, making it ideal for applications in transparent conductive electrodes and gas sensors. Tin sulfide (SnS), with a narrower bandgap and strong light absorption, shows promising semiconductor properties for photovoltaic devices but has lower electrical conductivity compared to tin oxide. The distinct optical bandgaps--approximately 3.6 eV for SnO2 and 1.3-1.5 eV for SnS--directly influence their suitability for transparent electronics versus solar energy harvesting.
Applications in Industry: Tin Oxide vs Tin Sulfide
Tin oxide (SnO2) is widely used in gas sensors, transparent conductive films, and ceramics due to its excellent electrical conductivity and chemical stability. Tin sulfide (SnS), known for its favorable optoelectronic properties, finds applications in photovoltaic cells, photodetectors, and thin-film semiconductors. Industrial use of tin oxide dominates in energy-efficient window coatings and catalytic converters, whereas tin sulfide's role is expanding in cost-effective solar energy harvesting and next-generation electronic devices.
Environmental Stability and Durability
Tin oxide (SnO2) exhibits superior environmental stability and durability compared to tin sulfide (SnS), as it resists oxidation and moisture-induced degradation more effectively. The robust nature of tin oxide's crystalline structure contributes to its enhanced resistance to corrosion and thermal stress, making it ideal for long-term applications in harsh environments. In contrast, tin sulfide tends to degrade faster under ambient conditions due to its susceptibility to photooxidation and moisture, limiting its lifespan and reliability in practical uses.
Cost and Availability
Tin oxide is more widely available and cost-effective due to its abundant natural sources and established industrial production methods, making it a common choice for various applications. Tin sulfide, on the other hand, tends to be more expensive and less readily available because of its complex synthesis processes and limited raw material sources. The cost disparity significantly influences their selection in manufacturing, with tin oxide preferred for budget-sensitive projects requiring scalable supply chains.
Advantages and Limitations
Tin oxide offers high electrical conductivity and excellent chemical stability, making it ideal for applications in transparent conductive films and gas sensors. Tin sulfide provides superior photovoltaic properties and eco-friendliness, suitable for solar cell applications due to its abundance and non-toxicity. Limitations of tin oxide include brittleness and higher production costs, while tin sulfide suffers from lower conductivity and stability issues under operational conditions.
Future Trends and Research Directions
Tin oxide (SnO2) and tin sulfide (SnS) research increasingly focuses on optimizing their electronic and optical properties for sustainable energy applications like photovoltaics and sensors. Future trends emphasize developing nanostructured composites and doping strategies to enhance charge transport and stability, addressing limitations in current device efficiencies. Exploring environmentally friendly synthesis methods and integrating these materials into flexible electronics remain critical research directions driving innovation in next-generation optoelectronic technologies.
Tin oxide vs tin sulfide Infographic
 materialdif.com
materialdif.com