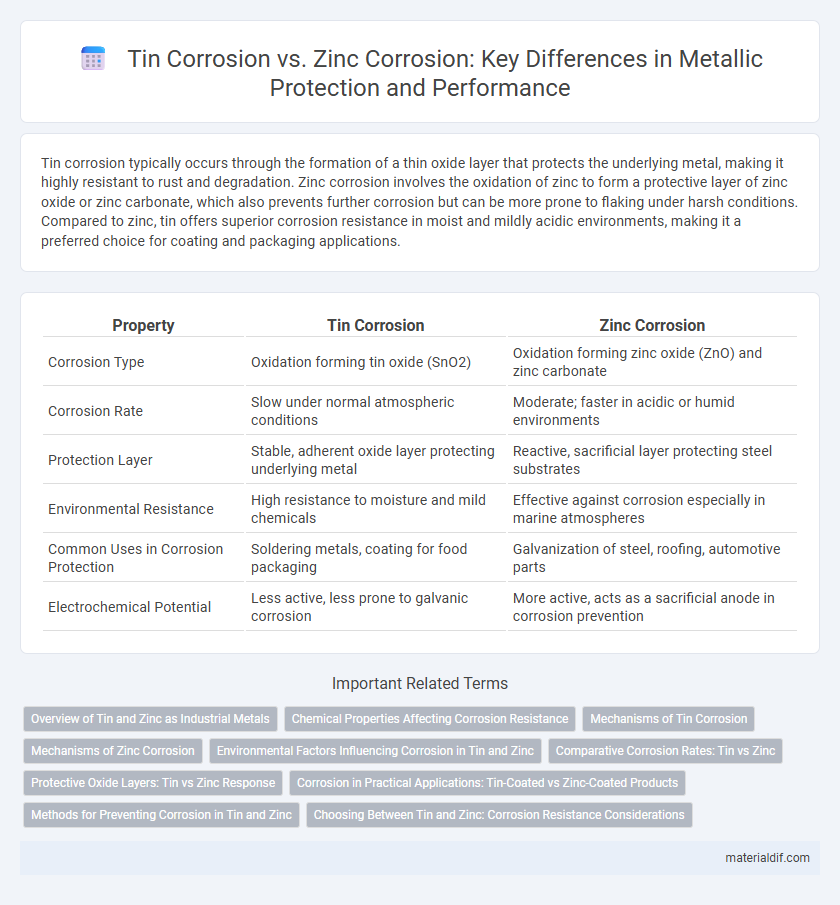
Tin corrosion typically occurs through the formation of a thin oxide layer that protects the underlying metal, making it highly resistant to rust and degradation. Zinc corrosion involves the oxidation of zinc to form a protective layer of zinc oxide or zinc carbonate, which also prevents further corrosion but can be more prone to flaking under harsh conditions. Compared to zinc, tin offers superior corrosion resistance in moist and mildly acidic environments, making it a preferred choice for coating and packaging applications.
Table of Comparison
| Property | Tin Corrosion | Zinc Corrosion |
|---|---|---|
| Corrosion Type | Oxidation forming tin oxide (SnO2) | Oxidation forming zinc oxide (ZnO) and zinc carbonate |
| Corrosion Rate | Slow under normal atmospheric conditions | Moderate; faster in acidic or humid environments |
| Protection Layer | Stable, adherent oxide layer protecting underlying metal | Reactive, sacrificial layer protecting steel substrates |
| Environmental Resistance | High resistance to moisture and mild chemicals | Effective against corrosion especially in marine atmospheres |
| Common Uses in Corrosion Protection | Soldering metals, coating for food packaging | Galvanization of steel, roofing, automotive parts |
| Electrochemical Potential | Less active, less prone to galvanic corrosion | More active, acts as a sacrificial anode in corrosion prevention |
Overview of Tin and Zinc as Industrial Metals
Tin exhibits excellent corrosion resistance, particularly in preventing oxidation and corrosion in electronics and food packaging, due to its stable oxide layer. Zinc, widely used for galvanization, offers superior sacrificial protection against corrosion by preferentially corroding to protect underlying steel structures. Both metals play crucial industrial roles, with tin favored for its non-toxic properties and zinc valued for its robust anticorrosive capabilities in structural applications.
Chemical Properties Affecting Corrosion Resistance
Tin exhibits superior corrosion resistance compared to zinc due to its stable oxide layer formation, which acts as an effective barrier against environmental factors. Zinc's higher electrochemical activity leads to accelerated corrosion, especially in acidic and marine environments, where zinc corrodes preferentially as a sacrificial anode. The chemical inertness and lower standard electrode potential of tin contribute to its enhanced durability in corrosive conditions relative to zinc.
Mechanisms of Tin Corrosion
Tin corrosion primarily occurs through oxidation, forming a stable tin oxide (SnO2) layer that acts as a protective barrier against further decay. Unlike zinc, which corrodes by forming zinc oxide and zinc carbonate in moist environments, tin's corrosion mechanism involves less aggressive electrochemical reactions, resulting in slower degradation. The passive oxide film on tin is less soluble and more adherent than the corrosion products formed on zinc, leading to superior resistance in various atmospheric and aqueous conditions.
Mechanisms of Zinc Corrosion
Zinc corrosion primarily occurs through a galvanic process where zinc acts as a sacrificial anode, protecting underlying metals by preferentially oxidizing to form zinc oxide or zinc carbonate layers. The formation of these corrosion products creates a protective barrier that slows further degradation. In contrast, tin corrosion involves uniform oxidation, generally resulting in tin oxide formation, which is less protective and leads to different degradation patterns compared to zinc.
Environmental Factors Influencing Corrosion in Tin and Zinc
Environmental factors such as humidity, temperature, and the presence of acidic or alkaline compounds critically influence the corrosion rates of tin and zinc. Tin generally exhibits better corrosion resistance in mildly acidic environments but can suffer from oxidation in high-humidity or chloride-rich conditions, whereas zinc forms a protective zinc carbonate layer in atmospheric exposure, enhancing its corrosion resistance. Industrial pollutants like sulfur dioxide accelerate corrosion in both metals, with zinc being more susceptible to degradation under highly acidic or alkaline environments.
Comparative Corrosion Rates: Tin vs Zinc
Tin exhibits significantly lower corrosion rates compared to zinc due to its greater resistance to oxidation and the formation of stable, protective oxide layers. Zinc corrodes more rapidly in most environments, especially in acidic or alkaline conditions, as its oxide layer is less adherent and more soluble. This difference in corrosion behavior makes tin a preferred choice for protective coatings where long-term durability against oxidation is critical.
Protective Oxide Layers: Tin vs Zinc Response
Tin forms a stable and adherent oxide layer (SnO2) that provides excellent corrosion resistance by acting as a protective barrier against moisture and atmospheric gases. Zinc, on the other hand, develops a less dense and more porous oxide layer (ZnO), which offers sacrificial protection by corroding preferentially to the underlying metal. The superior compactness of tin's oxide film results in enhanced durability and longer-lasting protection compared to zinc's oxide, especially in humid or marine environments.
Corrosion in Practical Applications: Tin-Coated vs Zinc-Coated Products
Tin-coated products exhibit superior corrosion resistance in environments with exposure to moisture and mild acids due to the formation of a stable, protective oxide layer, making them ideal for food packaging and electronic components. Zinc-coated steel, or galvanized steel, provides sacrificial protection by corroding preferentially to the underlying metal, which is advantageous in outdoor construction and automotive applications where weathering and mechanical damage occur. Both coatings enhance durability, but tin is preferred for applications requiring non-toxic surfaces and long-term resistance to mild corrosion, while zinc is favored for structural protection against harsh environmental conditions.
Methods for Preventing Corrosion in Tin and Zinc
Tin corrosion prevention relies heavily on protective coatings such as lacquers and organics, along with cathodic protection to reduce oxidation rates. Zinc corrosion is effectively minimized through galvanization, where zinc acts as a sacrificial anode, providing barrier protection and inhibiting rust formation on iron surfaces. Regular maintenance, use of inhibitors, and alloying elements also enhance corrosion resistance in both tin and zinc applications.
Choosing Between Tin and Zinc: Corrosion Resistance Considerations
Tin offers superior corrosion resistance compared to zinc, particularly in environments prone to moisture and acidic exposure, due to its stable oxide layer that prevents further oxidation. Zinc tends to corrode faster through galvanic corrosion when in contact with other metals, making it less suitable for harsh or marine environments. Selecting tin over zinc enhances durability and longevity for coatings and plating applications requiring robust corrosion protection.
Tin corrosion vs Zinc corrosion Infographic
 materialdif.com
materialdif.com