Static corrosion in tin occurs when the metal remains in a stationary environment, leading to surface degradation primarily due to prolonged exposure to moisture and oxygen. Dynamic corrosion involves tin subjected to mechanical movement or friction, accelerating wear and the formation of tin oxide layers that compromise the metal's integrity. Understanding the distinctions between static and dynamic corrosion helps in selecting appropriate protective coatings and maintenance strategies for tin-based applications.
Table of Comparison
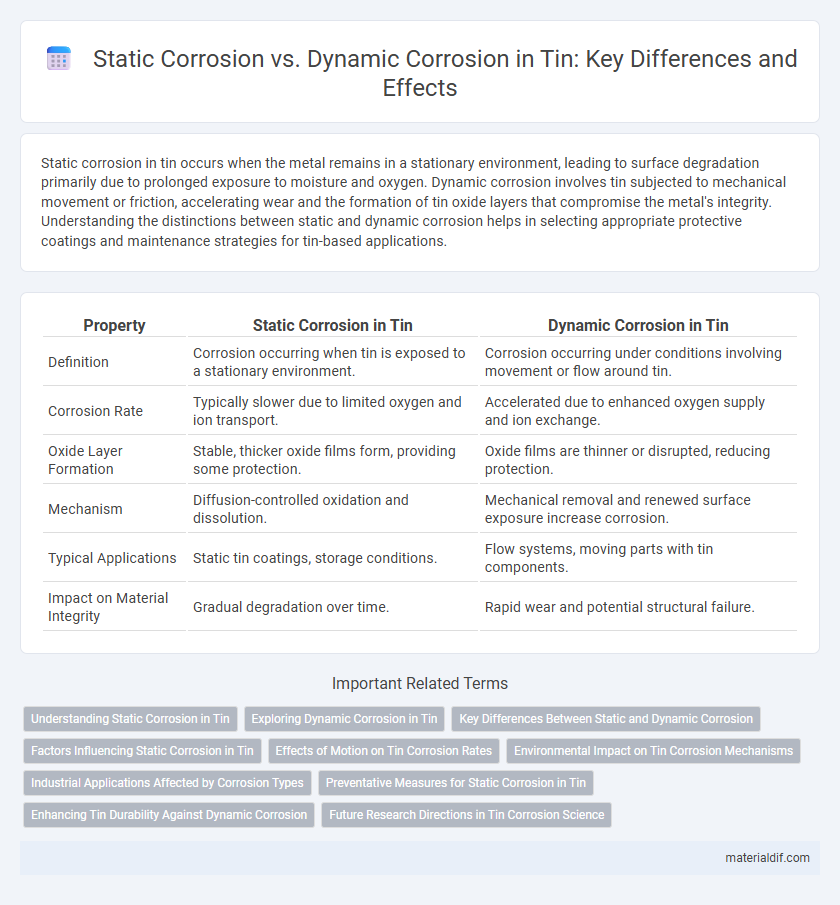
| Property | Static Corrosion in Tin | Dynamic Corrosion in Tin |
|---|---|---|
| Definition | Corrosion occurring when tin is exposed to a stationary environment. | Corrosion occurring under conditions involving movement or flow around tin. |
| Corrosion Rate | Typically slower due to limited oxygen and ion transport. | Accelerated due to enhanced oxygen supply and ion exchange. |
| Oxide Layer Formation | Stable, thicker oxide films form, providing some protection. | Oxide films are thinner or disrupted, reducing protection. |
| Mechanism | Diffusion-controlled oxidation and dissolution. | Mechanical removal and renewed surface exposure increase corrosion. |
| Typical Applications | Static tin coatings, storage conditions. | Flow systems, moving parts with tin components. |
| Impact on Material Integrity | Gradual degradation over time. | Rapid wear and potential structural failure. |
Understanding Static Corrosion in Tin
Static corrosion in tin primarily occurs when the metal is exposed to atmospheric oxygen and moisture without mechanical disturbance, causing the formation of a stable tin oxide layer on the surface. This passivation layer slows further corrosion by acting as a protective barrier, maintaining the metal's integrity over time. Factors such as temperature, humidity, and the presence of pollutants influence the rate and extent of static corrosion in tin alloys.
Exploring Dynamic Corrosion in Tin
Dynamic corrosion in tin involves material degradation accelerated by mechanical movement or fluid flow, which disrupts protective oxide layers and exposes fresh metal surfaces to corrosive agents. This form of corrosion is particularly significant in applications where tin-coatings experience vibrations or flowing electrolytes, leading to pitting and increased wear compared to static conditions. Understanding dynamic corrosion mechanisms in tin is essential for improving the durability of tin-plated components in electronics, automotive, and industrial systems.
Key Differences Between Static and Dynamic Corrosion
Static corrosion in tin primarily occurs through prolonged exposure to corrosive environments without movement, leading to uniform surface degradation often characterized by tin oxide formation. Dynamic corrosion involves tin in motion relative to its environment or other materials, causing accelerated wear, pitting, and localized corrosion due to friction and mechanical stresses. The key differences between static and dynamic corrosion in tin lie in their mechanisms, with static corrosion being chemical and time-dependent, while dynamic corrosion combines mechanical action with chemical reactions, resulting in faster material deterioration.
Factors Influencing Static Corrosion in Tin
Static corrosion in tin is primarily influenced by environmental factors such as humidity, temperature, and exposure to pollutants like sulfur compounds, which accelerate oxidation on tin surfaces. The presence of moisture creates an electrochemical environment that promotes the formation of tin oxide layers, leading to material degradation over time. Grain structure and alloy composition also play significant roles, affecting the uniformity and protective properties of the corrosion layer in static conditions.
Effects of Motion on Tin Corrosion Rates
The corrosion rate of tin significantly increases under dynamic conditions compared to static environments due to enhanced mass transport and exposure to corrosive agents. Motion accelerates the removal of protective oxide layers on tin surfaces, promoting faster degradation and pitting corrosion. Studies show that fluid velocity, turbulence, and mechanical abrasion directly influence tin's susceptibility to dynamic corrosion, impacting its durability in applications like heat exchangers and electronic components.
Environmental Impact on Tin Corrosion Mechanisms
Static corrosion in tin primarily occurs due to prolonged exposure to static environmental conditions, such as constant humidity and temperature, leading to uniform oxide layer formation. Dynamic corrosion involves fluctuating environments, including varying humidity, temperature, and mechanical stress, accelerating localized corrosion and pitting on tin surfaces. Environmental factors like pollutant presence, moisture levels, and temperature cycles significantly influence the corrosion mechanisms, affecting tin's durability and performance in applications such as electronics and packaging.
Industrial Applications Affected by Corrosion Types
Static corrosion in tin typically occurs in industrial environments with stagnant conditions, leading to uniform surface degradation affecting electronic components and food packaging. Dynamic corrosion involves tin exposure to moving fluids or mechanical wear, causing localized pitting or erosion in heat exchangers and automotive radiators. Both corrosion types critically impact the lifespan and reliability of tin-coated industrial equipment, necessitating tailored corrosion protection strategies.
Preventative Measures for Static Corrosion in Tin
Preventative measures for static corrosion in tin primarily involve controlling environmental factors such as humidity and exposure to corrosive agents like sulfur compounds and acids. Applying protective coatings, such as lacquers or polymer films, creates a barrier that significantly reduces the rate of oxidation and surface degradation. Regular inspection and maintenance, along with proper storage in low-humidity conditions, are critical to minimizing static corrosion and extending the metal's lifespan.
Enhancing Tin Durability Against Dynamic Corrosion
Tin exhibits significantly different behaviors under static corrosion and dynamic corrosion conditions, with dynamic corrosion posing greater challenges due to the combined effects of mechanical stress and chemical reactions. Enhancing tin durability against dynamic corrosion involves surface treatments such as alloying with elements like silver or copper, which improve mechanical strength and corrosion resistance simultaneously. Advanced coating technologies, including electroplating and passivation layers, further protect tin components by minimizing exposure to corrosive environments and reducing wear under dynamic stress.
Future Research Directions in Tin Corrosion Science
Future research in tin corrosion should focus on understanding the mechanistic differences between static and dynamic corrosion processes under various environmental and mechanical stress conditions. Advanced analytical techniques such as in situ electrochemical impedance spectroscopy and atomic force microscopy can elucidate the nanoscale interactions responsible for corrosion initiation and propagation. Developing predictive models integrating data from real-time monitoring of static and dynamic tin corrosion will enhance the design of more resilient tin-based materials and protective coatings.
Static Corrosion vs Dynamic Corrosion in Tin Infographic
 materialdif.com
materialdif.com