Vulcanization is a chemical process that strengthens rubber by creating cross-links between polymer chains, enhancing its elasticity, durability, and resistance to temperature and wear. Devulcanization reverses this process, breaking the sulfur bonds to restore rubber's original properties, enabling recycling and reuse of vulcanized rubber materials. Understanding the differences between vulcanization and devulcanization is essential for efficient rubber manufacturing and sustainable waste management.
Table of Comparison
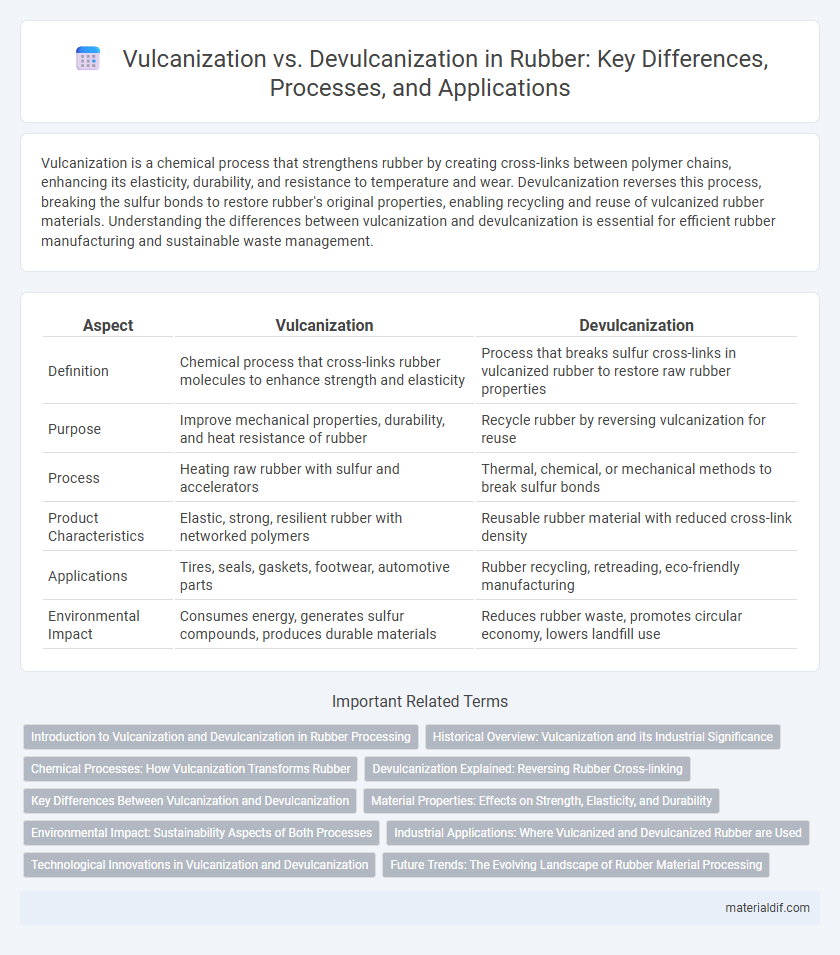
| Aspect | Vulcanization | Devulcanization |
|---|---|---|
| Definition | Chemical process that cross-links rubber molecules to enhance strength and elasticity | Process that breaks sulfur cross-links in vulcanized rubber to restore raw rubber properties |
| Purpose | Improve mechanical properties, durability, and heat resistance of rubber | Recycle rubber by reversing vulcanization for reuse |
| Process | Heating raw rubber with sulfur and accelerators | Thermal, chemical, or mechanical methods to break sulfur bonds |
| Product Characteristics | Elastic, strong, resilient rubber with networked polymers | Reusable rubber material with reduced cross-link density |
| Applications | Tires, seals, gaskets, footwear, automotive parts | Rubber recycling, retreading, eco-friendly manufacturing |
| Environmental Impact | Consumes energy, generates sulfur compounds, produces durable materials | Reduces rubber waste, promotes circular economy, lowers landfill use |
Introduction to Vulcanization and Devulcanization in Rubber Processing
Vulcanization is a chemical process that enhances rubber by forming cross-links between polymer chains, significantly improving its elasticity, strength, and durability. Devulcanization involves breaking these cross-links to recycle rubber, restoring its plasticity and allowing reuse in new products. Both processes are crucial in rubber manufacturing and recycling, optimizing material performance and sustainability.
Historical Overview: Vulcanization and its Industrial Significance
Vulcanization, discovered by Charles Goodyear in 1839, revolutionized the rubber industry by enhancing the material's elasticity, strength, and durability through the formation of sulfur cross-links between polymer chains. This chemical process transformed natural rubber into a commercially viable material widely used in automotive tires, industrial seals, and footwear, driving industrial growth in the 19th and 20th centuries. Devulcanization, developed later as a recycling technology, enables the breakdown of these sulfur bonds to reclaim rubber for secondary applications, addressing environmental concerns from rubber waste.
Chemical Processes: How Vulcanization Transforms Rubber
Vulcanization chemically transforms rubber by creating cross-links between polymer chains through sulfur atoms, enhancing elasticity, strength, and durability. This process involves heating raw rubber with sulfur, initiating a sulfur bridge formation that stabilizes the rubber's molecular structure. In contrast, devulcanization breaks these sulfur cross-links, restoring the rubber's original properties for recycling and reuse.
Devulcanization Explained: Reversing Rubber Cross-linking
Devulcanization is the chemical process that reverses vulcanization by breaking the sulfur cross-links in cured rubber, restoring its original elasticity and recyclability. This method enables recycled rubber to regain properties similar to virgin material, reducing waste and environmental impact. Industrial devulcanization techniques include thermal, chemical, and ultrasonic treatments, each targeting the selective cleavage of sulfur bonds while preserving polymer chains.
Key Differences Between Vulcanization and Devulcanization
Vulcanization is a chemical process that enhances rubber's elasticity and strength by creating cross-links between polymer chains, improving its durability and resistance to heat and wear. Devulcanization, on the other hand, reverses this process by breaking these cross-links, enabling the recycling and reuse of rubber materials without significant loss of properties. The primary difference lies in vulcanization's role in transforming raw rubber into a more robust material, while devulcanization serves to reclaim and restore used rubber for sustainable applications.
Material Properties: Effects on Strength, Elasticity, and Durability
Vulcanization enhances rubber's strength, elasticity, and durability by forming sulfur cross-links that improve resistance to heat and mechanical stress. Devulcanization reverses these cross-links to restore rubber's original flexibility, but often results in reduced tensile strength and lower durability compared to vulcanized rubber. Optimizing vulcanization parameters is crucial for achieving desired performance characteristics in automotive tires, seals, and industrial rubber products.
Environmental Impact: Sustainability Aspects of Both Processes
Vulcanization enhances rubber durability by creating cross-linked polymers, which extends product life and reduces waste generation. Devulcanization enables rubber recycling by breaking these cross-links, allowing reclaimed materials to be reused, thus minimizing landfill accumulation and raw material extraction. Both processes influence environmental sustainability, with devulcanization playing a critical role in circular economy practices for rubber products.
Industrial Applications: Where Vulcanized and Devulcanized Rubber are Used
Vulcanized rubber is predominantly used in automotive tires, conveyor belts, hoses, and industrial seals due to its enhanced strength, elasticity, and durability. Devulcanized rubber finds applications in rubber recycling industries, molded rubber products, and as reclaimed rubber in asphalt modification and rubber mats, promoting sustainability and cost efficiency. Both processes play critical roles in manufacturing sectors, optimizing material performance and resource reuse.
Technological Innovations in Vulcanization and Devulcanization
Technological innovations in vulcanization have advanced with the development of microwave and ultrasonic techniques, enhancing cross-linking efficiency and reducing processing time. In devulcanization, novel methods such as bio-devulcanization using enzymatic treatments and advanced chemical processes allow selective breaking of sulfur bonds, enabling effective rubber recycling. These innovations drive sustainability by improving material performance and enabling circular rubber economies.
Future Trends: The Evolving Landscape of Rubber Material Processing
Vulcanization enhances rubber durability by cross-linking polymer chains with sulfur, creating strong, heat-resistant materials essential for automotive and industrial applications. Devulcanization reverses this process, breaking sulfur bonds to recycle and soften rubber, promoting sustainability and resource efficiency in rubber manufacturing. Future trends emphasize advanced devulcanization technologies utilizing chemical, ultrasonic, and microwave methods to enable high-quality rubber recycling and reduce environmental impact.
Vulcanization vs Devulcanization Infographic
 materialdif.com
materialdif.com