Polyether and polyester are two primary types of polymers with distinct chemical structures and properties. Polyethers are known for their flexibility, excellent chemical resistance, and low glass transition temperatures, making them ideal for applications like elastomers and adhesives. Polyesters offer higher tensile strength, better abrasion resistance, and superior thermal stability, commonly used in fibers, films, and plastic containers.
Table of Comparison
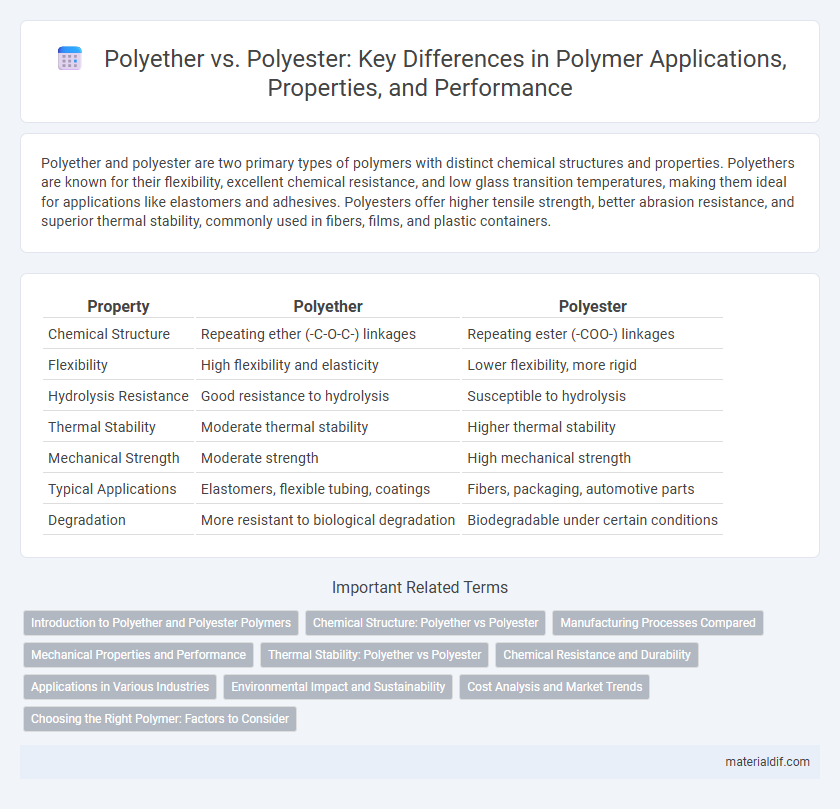
| Property | Polyether | Polyester |
|---|---|---|
| Chemical Structure | Repeating ether (-C-O-C-) linkages | Repeating ester (-COO-) linkages |
| Flexibility | High flexibility and elasticity | Lower flexibility, more rigid |
| Hydrolysis Resistance | Good resistance to hydrolysis | Susceptible to hydrolysis |
| Thermal Stability | Moderate thermal stability | Higher thermal stability |
| Mechanical Strength | Moderate strength | High mechanical strength |
| Typical Applications | Elastomers, flexible tubing, coatings | Fibers, packaging, automotive parts |
| Degradation | More resistant to biological degradation | Biodegradable under certain conditions |
Introduction to Polyether and Polyester Polymers
Polyether polymers, characterized by their ether linkages (-C-O-C-) in the polymer backbone, exhibit excellent flexibility, low glass transition temperatures, and high chemical resistance, making them ideal for applications like flexible foams and elastomers. In contrast, polyester polymers contain ester linkages (-COO-) and are known for their high tensile strength, rigidity, and thermal stability, commonly used in fibers, packaging, and plastic bottles. Understanding the molecular structure differences between polyethers and polyesters is crucial in selecting the right polymer for specific industrial or consumer applications.
Chemical Structure: Polyether vs Polyester
Polyether polymers feature repeating units linked by ether (-O-) bonds, resulting in a flexible backbone with high resistance to hydrolysis and chemicals. Polyester polymers consist of ester (-COO-) linkages in their backbone, which introduces rigidity and typically leads to higher tensile strength but increased susceptibility to hydrolytic degradation. The distinct chemical structures directly affect their mechanical properties, thermal stability, and applications in industries such as textiles, packaging, and biomedical devices.
Manufacturing Processes Compared
Polyether polymers are typically produced through ring-opening polymerization of epoxides, which often results in flexible, low-viscosity materials suitable for polyurethane applications. Polyester manufacturing involves polycondensation reactions between diacids and diols, generally requiring higher temperatures and longer reaction times to achieve high molecular weight polymers with strong mechanical properties. These distinct processes influence the thermal stability, chemical resistance, and application spectrum of polyethers versus polyesters in industrial production.
Mechanical Properties and Performance
Polyether polymers exhibit superior flexibility, impact resistance, and low-temperature performance compared to polyesters, making them ideal for applications requiring durability under dynamic stress. Polyester materials generally provide higher tensile strength, stiffness, and thermal stability, favoring structural and high-load environments. The choice between polyether and polyester depends on the balance between mechanical toughness and rigid performance needed for specific engineering applications.
Thermal Stability: Polyether vs Polyester
Polyesters generally exhibit higher thermal stability than polyethers due to their rigid aromatic backbone and stronger intermolecular hydrogen bonding, enabling them to maintain mechanical properties at elevated temperatures. Polyethers, with their flexible ether linkages, tend to have lower melting points and thermal degradation temperatures, limiting their use in high-temperature applications. The thermal decomposition of polyesters typically occurs above 300degC, while polyethers often degrade around 200-250degC, making polyesters preferable for heat-resistant polymer engineering.
Chemical Resistance and Durability
Polyether polymers exhibit superior chemical resistance to acids, bases, and solvents compared to polyesters, making them ideal for harsh chemical environments. Polyester materials offer excellent mechanical strength and UV durability but are more susceptible to hydrolysis and chemical degradation over time. The choice between polyether and polyester depends on the application's exposure to chemicals and the required lifespan under stress conditions.
Applications in Various Industries
Polyether resins are extensively used in the automotive and aerospace industries for their flexibility and resistance to hydrolysis, making them ideal for fuel lines and seals. Polyester resins are favored in construction and marine applications due to their excellent mechanical strength and resistance to environmental degradation, such as UV exposure and moisture. Both polymers serve crucial roles in electronics, with polyethers providing insulation and polyesters ensuring durability in circuit boards and components.
Environmental Impact and Sustainability
Polyether polymers typically exhibit lower biodegradability compared to polyesters, resulting in longer environmental persistence and potential accumulation in ecosystems. Polyester polymers, especially those derived from renewable resources like polylactic acid (PLA), demonstrate enhanced biodegradability and a reduced carbon footprint, contributing to improved sustainability profiles. Recycling processes for polyesters are more established and efficient, supporting circular economy initiatives and lowering overall environmental impact.
Cost Analysis and Market Trends
Polyether polymers typically incur higher production costs due to their specialized manufacturing processes and raw materials, but they offer superior flexibility and chemical resistance sought in niche markets. Polyester polymers, benefiting from established large-scale production and abundant feedstock, maintain a cost advantage that drives widespread use in textile, packaging, and automotive industries. Market trends indicate growing demand for bio-based polyesters driven by sustainability goals, while polyether demand remains steady in high-performance sectors requiring durability and thermal stability.
Choosing the Right Polymer: Factors to Consider
Selecting between polyether and polyester depends on factors such as chemical resistance, flexibility, and mechanical strength requirements. Polyethers offer superior hydrolytic stability and elasticity, making them ideal for applications involving moisture exposure and dynamic stresses. Polyesters provide enhanced tensile strength and resistance to UV degradation, suitable for structural components and outdoor usage.
Polyether vs Polyester Infographic
 materialdif.com
materialdif.com