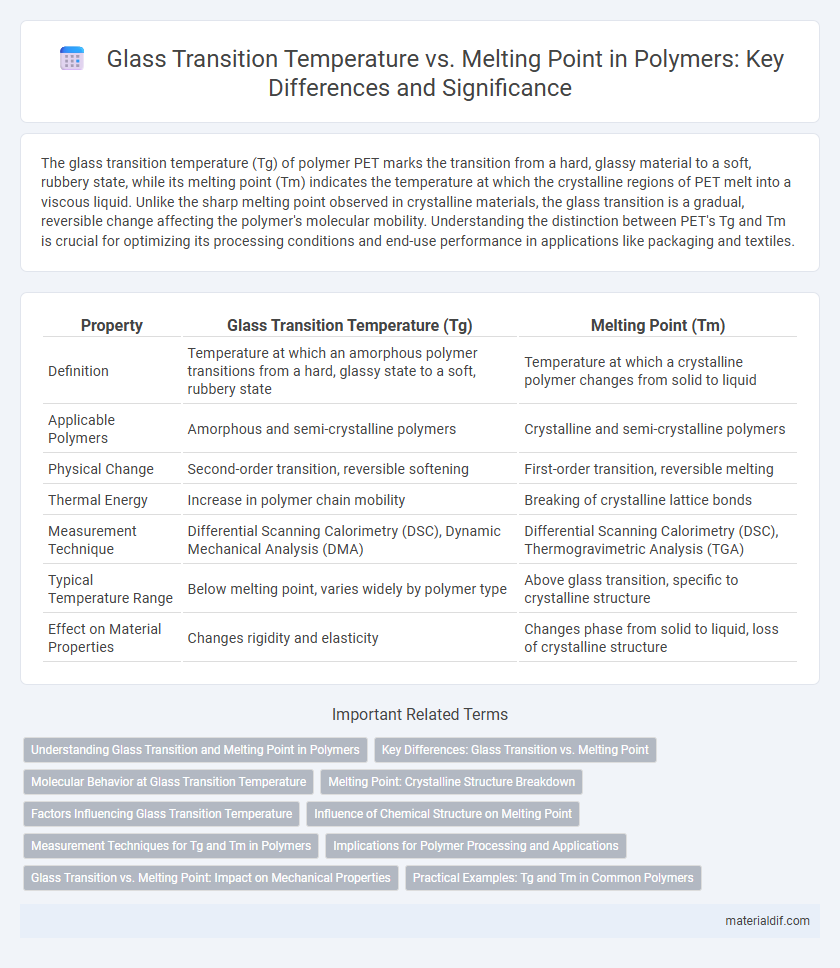
The glass transition temperature (Tg) of polymer PET marks the transition from a hard, glassy material to a soft, rubbery state, while its melting point (Tm) indicates the temperature at which the crystalline regions of PET melt into a viscous liquid. Unlike the sharp melting point observed in crystalline materials, the glass transition is a gradual, reversible change affecting the polymer's molecular mobility. Understanding the distinction between PET's Tg and Tm is crucial for optimizing its processing conditions and end-use performance in applications like packaging and textiles.
Table of Comparison
| Property | Glass Transition Temperature (Tg) | Melting Point (Tm) |
|---|---|---|
| Definition | Temperature at which an amorphous polymer transitions from a hard, glassy state to a soft, rubbery state | Temperature at which a crystalline polymer changes from solid to liquid |
| Applicable Polymers | Amorphous and semi-crystalline polymers | Crystalline and semi-crystalline polymers |
| Physical Change | Second-order transition, reversible softening | First-order transition, reversible melting |
| Thermal Energy | Increase in polymer chain mobility | Breaking of crystalline lattice bonds |
| Measurement Technique | Differential Scanning Calorimetry (DSC), Dynamic Mechanical Analysis (DMA) | Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA) |
| Typical Temperature Range | Below melting point, varies widely by polymer type | Above glass transition, specific to crystalline structure |
| Effect on Material Properties | Changes rigidity and elasticity | Changes phase from solid to liquid, loss of crystalline structure |
Understanding Glass Transition and Melting Point in Polymers
Glass transition in polymers refers to the temperature range where the polymer transitions from a hard, glassy material to a soft, rubbery state without melting. The melting point is a distinct, sharp temperature where crystalline regions in semi-crystalline polymers dissolve into a liquid phase. Understanding these thermal transitions is crucial for predicting polymer performance in applications involving temperature changes.
Key Differences: Glass Transition vs. Melting Point
The glass transition temperature (Tg) marks the reversible change of a polymer from a hard, glassy state to a soft, rubbery state without a phase change, while the melting point (Tm) indicates the temperature at which a crystalline polymer transitions from solid to liquid. Tg is characteristic of amorphous regions and involves changes in molecular mobility and free volume, whereas Tm is specific to crystalline domains and involves breaking of the ordered lattice. Understanding the distinction between Tg and Tm is critical for polymer processing and application, influencing mechanical properties and thermal stability.
Molecular Behavior at Glass Transition Temperature
At the glass transition temperature (Tg), polymer molecules undergo a shift from a rigid, glassy state to a more flexible, rubbery state characterized by increased segmental mobility without reaching the crystalline order observed at the melting point (Tm). Unlike melting, which involves the breakdown of crystalline structures, the molecular chains at Tg experience increased free volume and cooperative motion of chain segments, resulting in a drastic change in mechanical properties. This transition significantly influences polymer toughness, impact resistance, and viscoelastic behavior, critical for applications requiring specific thermal and mechanical performance.
Melting Point: Crystalline Structure Breakdown
The melting point of polymers signifies the temperature at which the crystalline regions break down, causing a transition from solid to liquid due to the disruption of ordered molecular chains. This phase change involves the complete breakdown of the crystalline structure, resulting in loss of mechanical strength and shape retention. Unlike the glass transition temperature, which affects the amorphous regions, the melting point specifically marks the destruction of the polymer's crystalline lattice.
Factors Influencing Glass Transition Temperature
The glass transition temperature (Tg) of a polymer is influenced by factors such as polymer chain flexibility, molecular weight, and intermolecular forces like hydrogen bonding and van der Waals interactions. High molecular weight and strong intermolecular forces typically increase Tg by restricting polymer chain mobility, while increased chain flexibility lowers Tg. Unlike melting point (Tm), which depends on crystalline regions, Tg reflects the transition of amorphous polymer segments from a rigid to a rubbery state.
Influence of Chemical Structure on Melting Point
The chemical structure of polymers significantly influences their melting point by determining the degree of crystallinity and chain packing efficiency. Polymers with regular, symmetrical structures and strong intermolecular forces typically exhibit higher melting points due to enhanced chain alignment. In contrast, irregular or branched polymers disrupt crystallinity, lowering the melting point and affecting thermal processing characteristics.
Measurement Techniques for Tg and Tm in Polymers
Differential scanning calorimetry (DSC) is the most widely used technique to measure the glass transition temperature (Tg) and melting point (Tm) in polymers, detecting changes in heat flow associated with phase transitions. Dynamic mechanical analysis (DMA) provides complementary information on Tg by measuring the polymer's mechanical response, such as storage and loss moduli, over a temperature range. Thermomechanical analysis (TMA) and dielectric analysis (DEA) can also be employed to accurately determine Tg and Tm by monitoring dimensional changes or dielectric properties as the polymer undergoes thermal transitions.
Implications for Polymer Processing and Applications
The glass transition temperature (Tg) and melting point (Tm) critically influence polymer processing methods such as extrusion and injection molding by determining the temperature ranges for material softening and flow. Polymers processed near or above Tg exhibit increased molecular mobility without complete phase change, enabling flexibility in applications like packaging and adhesives, whereas processing above Tm facilitates crystalline polymer reshaping necessary for structural components. Understanding these thermal transitions allows engineers to tailor polymer selection for applications that require specific mechanical properties, thermal stability, or impact resistance.
Glass Transition vs. Melting Point: Impact on Mechanical Properties
Glass transition temperature (Tg) marks the transition of a polymer from a rigid, glassy state to a more flexible, rubbery state, significantly affecting its impact resistance and tensile strength. Melting point (Tm) defines the temperature at which crystalline regions of the polymer liquefy, influencing thermal stability and dimensional integrity under heat. Polymers with a distinct Tg below their Tm exhibit enhanced mechanical properties by balancing rigidity and flexibility, crucial for applications requiring both toughness and thermal resistance.
Practical Examples: Tg and Tm in Common Polymers
Polymers such as polystyrene exhibit a glass transition temperature (Tg) around 100degC, where the material changes from a hard, glassy state to a rubbery state without melting. In contrast, polyethylene has a melting point (Tm) near 130degC, representing the temperature at which the crystalline regions of the polymer melt and flow. Understanding Tg and Tm is crucial for applications like packaging, where polystyrene's Tg affects rigidity and polyethylene's Tm determines thermal processing limits.
Glass Transition vs Melting Point Infographic
 materialdif.com
materialdif.com