Co-polymerization combines two or more different monomers to create polymers with enhanced properties such as improved strength, flexibility, or chemical resistance, making it ideal for specialized applications. Homopolymerization involves polymerizing a single type of monomer, resulting in polymers with uniform structure and predictable performance characteristics, often used for standard plastic products. The choice between co-polymerization and homopolymerization depends on the desired material properties and application requirements in polymer production.
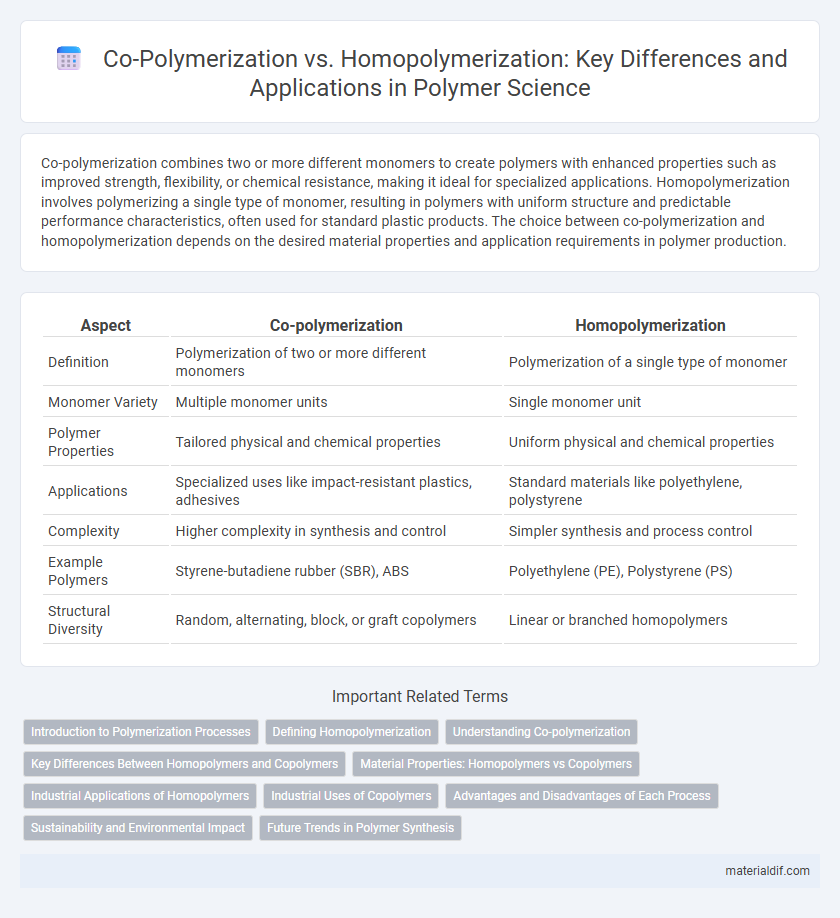
Table of Comparison
| Aspect | Co-polymerization | Homopolymerization |
|---|---|---|
| Definition | Polymerization of two or more different monomers | Polymerization of a single type of monomer |
| Monomer Variety | Multiple monomer units | Single monomer unit |
| Polymer Properties | Tailored physical and chemical properties | Uniform physical and chemical properties |
| Applications | Specialized uses like impact-resistant plastics, adhesives | Standard materials like polyethylene, polystyrene |
| Complexity | Higher complexity in synthesis and control | Simpler synthesis and process control |
| Example Polymers | Styrene-butadiene rubber (SBR), ABS | Polyethylene (PE), Polystyrene (PS) |
| Structural Diversity | Random, alternating, block, or graft copolymers | Linear or branched homopolymers |
Introduction to Polymerization Processes
Co-polymerization involves the polymerization of two or more different monomers to form a copolymer with tailored properties, whereas homopolymerization uses a single type of monomer resulting in uniform polymer chains. The choice between co-polymerization and homopolymerization significantly affects the physical, chemical, and mechanical characteristics of the resulting polymer. Understanding these polymerization processes is essential for designing materials with specific applications in mind, from flexible plastics to high-strength fibers.
Defining Homopolymerization
Homopolymerization is a polymerization process where only one type of monomer is repeatedly linked to form a polymer chain, resulting in a polymer composed of identical repeating units. This method yields polymers with uniform chemical and physical properties, such as polyethylene or polystyrene. The process is simpler compared to co-polymerization, which involves two or more different monomers, enabling tailored material characteristics.
Understanding Co-polymerization
Co-polymerization involves the polymerization of two different monomers, producing copolymers with tailored properties such as enhanced strength, flexibility, or chemical resistance that cannot be achieved with homopolymers formed from a single monomer. Understanding co-polymerization allows for the design of materials with specific functionalities by controlling monomer ratios, sequence distribution, and polymer architecture, optimizing performance for applications in industries like automotive, packaging, and biomedical devices. Precise control over reaction conditions and monomer compatibility is essential to achieve desired copolymer microstructures and properties.
Key Differences Between Homopolymers and Copolymers
Homopolymers consist of identical monomer units linked in a repeating sequence, resulting in uniform chemical and physical properties, while copolymers are formed from two or more different monomer species, enabling tunable characteristics and enhanced material performance. Key differences include molecular structure diversity, thermal behavior, mechanical strength, and chemical resistance, with copolymers offering improved flexibility and compatibility for specialized applications. The variation in monomer arrangement influences crystallinity and phase behavior, directly impacting the polymer's applicability and processing conditions.
Material Properties: Homopolymers vs Copolymers
Homopolymers consist of identical monomer units, resulting in uniform properties such as high crystallinity and consistent melting points, which provide mechanical strength and thermal stability. Copolymers incorporate two or more different monomer types, enabling tailored material properties like improved flexibility, impact resistance, or chemical resistance due to microstructural variation. The choice between homopolymerization and copolymerization directly influences polymer morphology, affecting applications ranging from packaging films to automotive components.
Industrial Applications of Homopolymers
Homopolymerization produces polymers from a single type of monomer, resulting in materials with consistent physical properties ideal for industrial applications such as polyethylene in packaging, polypropylene in automotive parts, and polystyrene in insulation. Homopolymers offer high purity and uniformity, promoting ease of processing and predictable performance in manufacturing sectors. Industrially, these polymers serve as essential raw materials in textiles, plastic containers, and construction, due to their durability, chemical resistance, and cost-effectiveness.
Industrial Uses of Copolymers
Copolymerization enables the production of materials with tailored properties by combining two or more monomers, offering enhanced flexibility, strength, and chemical resistance compared to homopolymers. Industrial applications of copolymers include automotive parts, packaging films, and medical devices, where specific performance characteristics like impact resistance and durability are critical. This versatility in copolymer design supports innovation in sectors demanding materials with precise mechanical and thermal properties.
Advantages and Disadvantages of Each Process
Co-polymerization offers enhanced material properties like improved strength, flexibility, and chemical resistance by combining different monomers, but it involves more complex synthesis and characterization compared to homopolymerization. Homopolymerization provides simpler reaction mechanisms and consistent polymer chains, resulting in predictable physical properties, though it limits the diversity of polymer functionalities and may produce materials with inferior mechanical or thermal performance. Selecting between co-polymerization and homopolymerization depends on the intended application requirements, balancing synthesis complexity against desired polymer characteristics.
Sustainability and Environmental Impact
Co-polymerization enhances sustainability by enabling the design of polymers with tailored properties such as improved biodegradability and reduced carbon footprint compared to homopolymers. The incorporation of renewable monomers in co-polymerization processes can reduce reliance on fossil fuels, leading to lower environmental impact during production and end-of-life degradation. Homopolymerization, while simpler, often results in materials with limited recyclability and higher environmental persistence, posing challenges for sustainable polymer lifecycle management.
Future Trends in Polymer Synthesis
Future trends in polymer synthesis emphasize co-polymerization techniques to create materials with enhanced mechanical properties and tailored functionalities compared to homopolymerization. Advances in controlled radical polymerization and ring-opening metathesis polymerization enable precise incorporation of diverse monomers, optimizing polymer performance for biomedical, automotive, and electronic applications. Emerging green chemistry approaches aim to develop sustainable co-polymers with improved biodegradability and reduced environmental impact.
Co-polymerization vs Homopolymerization Infographic
 materialdif.com
materialdif.com