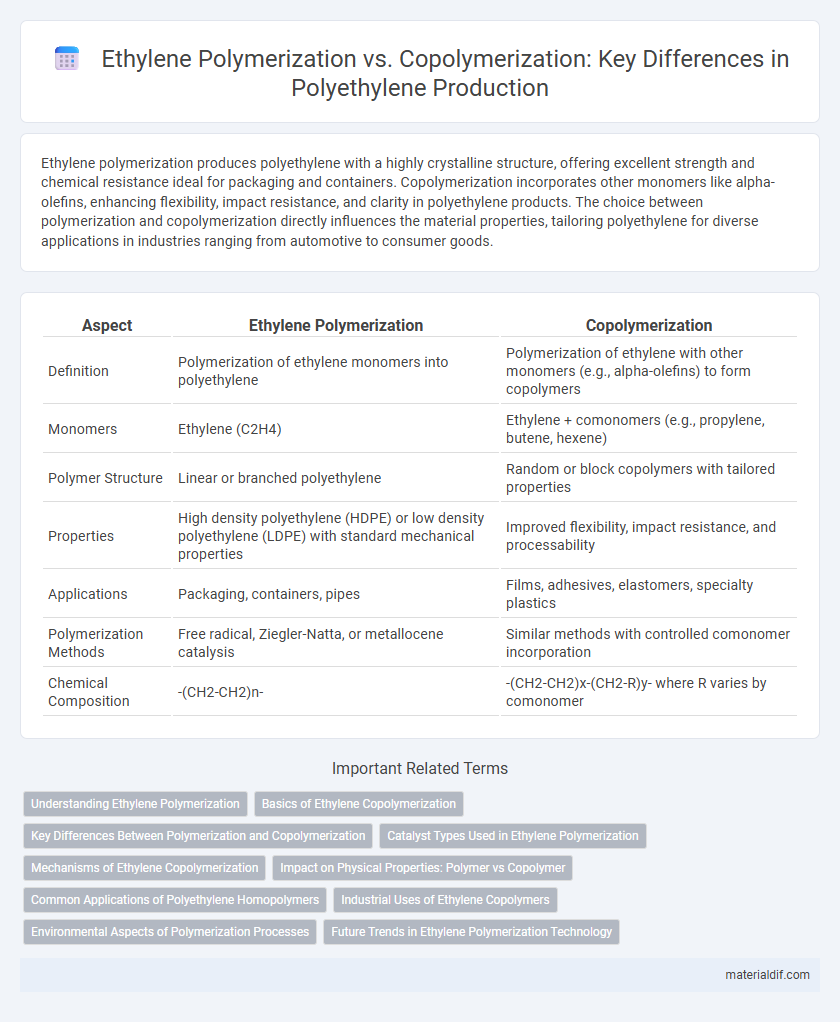
Ethylene polymerization produces polyethylene with a highly crystalline structure, offering excellent strength and chemical resistance ideal for packaging and containers. Copolymerization incorporates other monomers like alpha-olefins, enhancing flexibility, impact resistance, and clarity in polyethylene products. The choice between polymerization and copolymerization directly influences the material properties, tailoring polyethylene for diverse applications in industries ranging from automotive to consumer goods.
Table of Comparison
| Aspect | Ethylene Polymerization | Copolymerization |
|---|---|---|
| Definition | Polymerization of ethylene monomers into polyethylene | Polymerization of ethylene with other monomers (e.g., alpha-olefins) to form copolymers |
| Monomers | Ethylene (C2H4) | Ethylene + comonomers (e.g., propylene, butene, hexene) |
| Polymer Structure | Linear or branched polyethylene | Random or block copolymers with tailored properties |
| Properties | High density polyethylene (HDPE) or low density polyethylene (LDPE) with standard mechanical properties | Improved flexibility, impact resistance, and processability |
| Applications | Packaging, containers, pipes | Films, adhesives, elastomers, specialty plastics |
| Polymerization Methods | Free radical, Ziegler-Natta, or metallocene catalysis | Similar methods with controlled comonomer incorporation |
| Chemical Composition | -(CH2-CH2)n- | -(CH2-CH2)x-(CH2-R)y- where R varies by comonomer |
Understanding Ethylene Polymerization
Ethylene polymerization involves the catalytic conversion of ethylene monomers into high-density polyethylene (HDPE), emphasizing chain growth through coordination-insertion mechanisms using catalysts such as Ziegler-Natta or metallocenes. This process controls polymer properties like molecular weight and crystallinity by regulating reaction conditions, including temperature, pressure, and catalyst type. Copolymerization, in contrast, incorporates comonomers such as butene or hexene to modify polyethylene properties, but ethylene polymerization remains fundamental for producing uniform, high-purity polyethylene.
Basics of Ethylene Copolymerization
Ethylene copolymerization involves the reaction of ethylene with one or more different monomers to produce copolymers with modified properties compared to homopolymers formed by ethylene polymerization alone. Common comonomers include alpha-olefins like butene, hexene, and octene, which affect density, crystallinity, and mechanical strength of the resulting polyethylene. The catalytic systems, such as Ziegler-Natta or metallocene catalysts, play a crucial role in controlling the incorporation of comonomers, thereby tuning polymer architecture and performance characteristics.
Key Differences Between Polymerization and Copolymerization
Polymerization of ethylene produces homopolymers consisting solely of polyethylene chains, characterized by uniform molecular structure and properties. Copolymerization incorporates one or more comonomers, such as alpha-olefins, resulting in materials with modified density, crystallinity, and mechanical performance. The key differences lie in chemical composition variation, control over polymer architecture, and tunability of physical properties for specific applications.
Catalyst Types Used in Ethylene Polymerization
Ziegler-Natta catalysts, metallocene catalysts, and chromium-based catalysts dominate ethylene polymerization due to their efficiency in producing high-density polyethylene (HDPE) and linear low-density polyethylene (LLDPE). Ziegler-Natta catalysts are widely utilized for their ability to produce stereoregular polymers, while metallocene catalysts offer precise control over molecular weight distribution and polymer architecture. Chromium-based catalysts are favored for producing ultrahigh molecular weight polyethylene (UHMWPE) with excellent mechanical properties.
Mechanisms of Ethylene Copolymerization
Ethylene copolymerization involves the incorporation of comonomers such as 1-hexene or vinyl acetate into the polyethylene chain through coordination-insertion mechanisms facilitated by Ziegler-Natta or metallocene catalysts. The catalyst active site coordinates both ethylene and comonomer, enabling insertion into the growing polymer chain, which alters crystallinity, density, and mechanical properties. Control over comonomer incorporation and distribution during copolymerization impacts polymer morphology and performance, distinguishing it from homopolymerization of ethylene.
Impact on Physical Properties: Polymer vs Copolymer
Ethylene polymerization produces polyethylene with high crystallinity and density, resulting in superior tensile strength and rigidity. In contrast, copolymerization of ethylene with comonomers like hexene or butene disrupts the polymer chain regularity, leading to lower crystallinity and enhanced flexibility and impact resistance. The tailored balance between crystallinity and amorphous regions in copolymers allows for improved processability and application-specific mechanical properties compared to homopolymeric polyethylene.
Common Applications of Polyethylene Homopolymers
Polyethylene homopolymers, produced through ethylene polymerization, exhibit high density and crystallinity, making them ideal for applications such as plastic bags, bottles, and piping materials. Their robust chemical resistance and mechanical strength enable extensive use in packaging, containers, and geomembranes. Compared to copolymers, homopolymers offer greater rigidity and thermal stability, which are essential in industries requiring durable and stable polymer products.
Industrial Uses of Ethylene Copolymers
Ethylene copolymers, produced by incorporating comonomers like hexene or butene during ethylene polymerization, exhibit enhanced mechanical properties and flexibility compared to homopolymers. These copolymers find extensive industrial applications in packaging films, automotive parts, and medical devices due to their improved toughness, clarity, and impact resistance. The ability to tailor properties through copolymerization processes makes ethylene copolymers essential in manufacturing durable, lightweight, and versatile materials across multiple sectors.
Environmental Aspects of Polymerization Processes
Ethylene polymerization primarily produces homopolyethylene, known for its straightforward chemical structure and recyclability, which can facilitate more efficient recycling processes and reduce plastic waste accumulation. Copolymerization, involving ethylene and other monomers, produces materials with enhanced properties but may complicate recycling due to the diverse chemical compositions and potential for increased environmental persistence. The choice between polymerization and copolymerization impacts energy consumption, greenhouse gas emissions, and end-of-life management, influencing the overall environmental footprint of polyethylene production.
Future Trends in Ethylene Polymerization Technology
Future trends in ethylene polymerization technology emphasize advanced catalyst design to improve polymer structure control, enhancing material properties and process efficiency. Innovations in copolymerization enable the integration of polar monomers, expanding polyethylene applications in packaging and automotive industries. Sustainable approaches focus on energy-efficient processes and the incorporation of bio-based feedstocks to reduce environmental impact.
Ethylene Polymerization vs Copolymerization Infographic
 materialdif.com
materialdif.com