Corrosion resistance refers to the ability of metal to withstand damage caused by chemical reactions with environmental elements such as moisture, acids, and salts, preventing rust and degradation. Oxidation resistance specifically describes a metal's capability to resist oxidation, a chemical reaction where oxygen interacts with the metal surface, often forming an oxide layer that can either protect or further degrade the metal. While both properties enhance metal durability, corrosion resistance encompasses a broader range of chemical attacks beyond just oxidation.
Table of Comparison
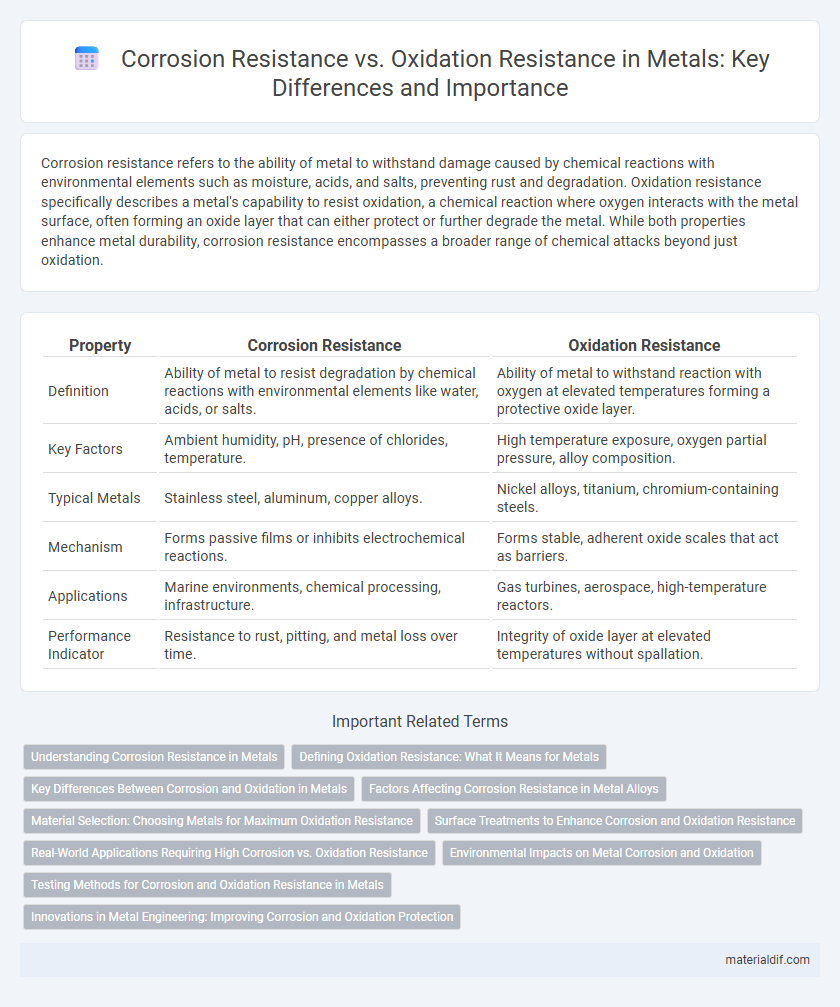
| Property | Corrosion Resistance | Oxidation Resistance |
|---|---|---|
| Definition | Ability of metal to resist degradation by chemical reactions with environmental elements like water, acids, or salts. | Ability of metal to withstand reaction with oxygen at elevated temperatures forming a protective oxide layer. |
| Key Factors | Ambient humidity, pH, presence of chlorides, temperature. | High temperature exposure, oxygen partial pressure, alloy composition. |
| Typical Metals | Stainless steel, aluminum, copper alloys. | Nickel alloys, titanium, chromium-containing steels. |
| Mechanism | Forms passive films or inhibits electrochemical reactions. | Forms stable, adherent oxide scales that act as barriers. |
| Applications | Marine environments, chemical processing, infrastructure. | Gas turbines, aerospace, high-temperature reactors. |
| Performance Indicator | Resistance to rust, pitting, and metal loss over time. | Integrity of oxide layer at elevated temperatures without spallation. |
Understanding Corrosion Resistance in Metals
Corrosion resistance in metals refers to the ability to withstand degradation caused by chemical or electrochemical reactions with the environment, primarily oxidation, moisture, and other corrosive agents. This property depends on the metal's composition, surface treatments, and protective coatings that inhibit the formation of rust or other corrosive products. Understanding corrosion resistance is critical for selecting materials in industries like construction, automotive, and marine engineering, where longevity and durability under harsh conditions are essential.
Defining Oxidation Resistance: What It Means for Metals
Oxidation resistance in metals refers to their ability to withstand chemical reactions with oxygen that cause surface degradation and loss of material integrity. This property is critical for metals used in high-temperature or corrosive environments, as it prevents the formation of oxides that compromise mechanical strength and appearance. Unlike general corrosion resistance, oxidation resistance specifically targets the metal's defense against oxidative processes, ensuring longer durability and improved performance in applications such as aerospace, automotive, and industrial machinery.
Key Differences Between Corrosion and Oxidation in Metals
Corrosion resistance in metals refers to the ability to withstand chemical or electrochemical reactions with environmental agents, often involving moisture, acids, or salts, leading to material degradation. Oxidation resistance specifically pertains to a metal's capacity to resist reaction with oxygen or oxygen-containing compounds, typically at elevated temperatures, which forms oxide layers on the metal surface. The key difference lies in corrosion being a broader electrochemical process that deteriorates metal integrity, while oxidation primarily involves the formation of oxides that can either protect or degrade the metal depending on the oxide's adherence and stability.
Factors Affecting Corrosion Resistance in Metal Alloys
Corrosion resistance in metal alloys depends heavily on factors such as alloy composition, environmental conditions, and surface treatments. Elements like chromium and nickel enhance the formation of stable oxide layers, improving corrosion resistance by preventing metal degradation. Temperature, pH levels, and exposure to moisture or chemicals also significantly influence the rate and extent of corrosion in metal alloys.
Material Selection: Choosing Metals for Maximum Oxidation Resistance
Selecting metals with high chromium and nickel content enhances oxidation resistance by forming stable, protective oxide layers at elevated temperatures. Alloys like stainless steel and nickel-based superalloys demonstrate superior performance in oxidative environments due to their ability to prevent further metal degradation. Prioritizing materials with proven oxidation resistance extends component lifespan and reduces maintenance in high-temperature applications.
Surface Treatments to Enhance Corrosion and Oxidation Resistance
Surface treatments such as anodizing, electroplating, and chemical passivation significantly enhance corrosion resistance by forming protective oxide layers on metal surfaces, reducing exposure to corrosive environments. Oxidation resistance is improved through coatings like ceramic or aluminized layers that act as barriers against high-temperature oxidation. Combining these treatments optimizes metal durability in harsh environments, extending lifespan and maintaining structural integrity.
Real-World Applications Requiring High Corrosion vs. Oxidation Resistance
Corrosion resistance is critical in marine and chemical processing industries where metals face prolonged exposure to moisture, acids, and salts, demanding materials like stainless steel and titanium alloys to prevent degradation. Oxidation resistance is essential in high-temperature environments such as aerospace and automotive engine components, where metals like superalloys and chromium-coated steels must withstand aggressive oxidation without losing structural integrity. Selecting materials based on specific resistance needs ensures longevity and safety in demanding real-world applications.
Environmental Impacts on Metal Corrosion and Oxidation
Corrosion resistance in metals is primarily influenced by environmental factors such as humidity, temperature, and the presence of corrosive agents like saltwater and industrial pollutants, which accelerate metal degradation. Oxidation resistance depends on the ability of a metal's surface to form a stable oxide layer that prevents further reaction, but aggressive environments rich in oxygen, moisture, and acidic compounds can compromise this protective barrier. Understanding the interplay of environmental conditions and metal properties is crucial for selecting materials that minimize structural damage and extend the lifespan of metal components in harsh settings.
Testing Methods for Corrosion and Oxidation Resistance in Metals
Testing methods for corrosion resistance in metals commonly include salt spray tests, such as ASTM B117, which simulate aggressive chloride environments to evaluate material degradation. Oxidation resistance is typically assessed through high-temperature oxidation tests, where metals are exposed to controlled atmospheres at elevated temperatures to measure scale formation and weight gain. Electrochemical impedance spectroscopy (EIS) and thermogravimetric analysis (TGA) provide precise data on corrosion kinetics and oxidation rates, enabling comprehensive material performance evaluation.
Innovations in Metal Engineering: Improving Corrosion and Oxidation Protection
Innovations in metal engineering have led to advanced coatings and alloy compositions that significantly enhance both corrosion resistance and oxidation resistance, extending the lifespan of critical metal components in harsh environments. Nanostructured coatings and self-healing materials provide superior protection by forming stable oxide layers that prevent further degradation. These technological advancements optimize metal performance in industries such as aerospace, automotive, and infrastructure, reducing maintenance costs and improving safety.
Corrosion Resistance vs Oxidation Resistance Infographic
 materialdif.com
materialdif.com