The Bessemer Process revolutionized iron production by rapidly blowing air through molten iron to remove impurities, enabling mass production of steel with high efficiency. In contrast, the Open Hearth Process allowed for more precise control of temperature and composition, producing higher-quality steel through slower, more refined melting using a regenerative furnace. Both methods significantly advanced the iron and steel industry but differed in speed, control, and quality output.
Table of Comparison
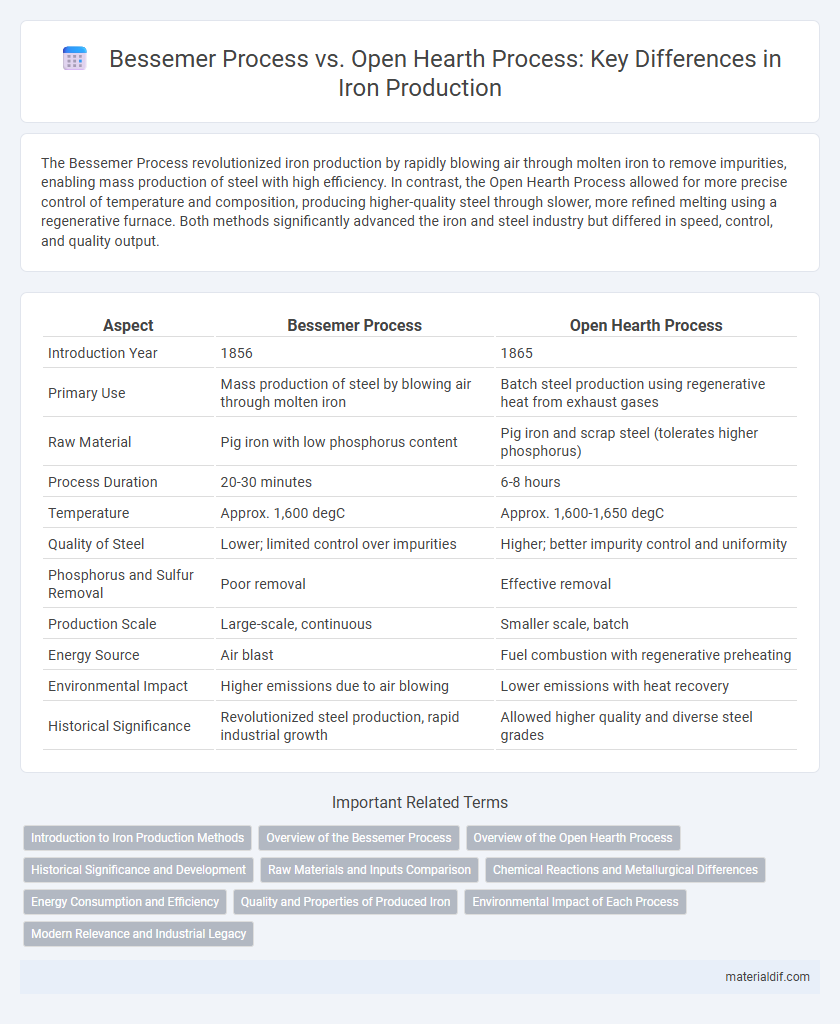
| Aspect | Bessemer Process | Open Hearth Process |
|---|---|---|
| Introduction Year | 1856 | 1865 |
| Primary Use | Mass production of steel by blowing air through molten iron | Batch steel production using regenerative heat from exhaust gases |
| Raw Material | Pig iron with low phosphorus content | Pig iron and scrap steel (tolerates higher phosphorus) |
| Process Duration | 20-30 minutes | 6-8 hours |
| Temperature | Approx. 1,600 degC | Approx. 1,600-1,650 degC |
| Quality of Steel | Lower; limited control over impurities | Higher; better impurity control and uniformity |
| Phosphorus and Sulfur Removal | Poor removal | Effective removal |
| Production Scale | Large-scale, continuous | Smaller scale, batch |
| Energy Source | Air blast | Fuel combustion with regenerative preheating |
| Environmental Impact | Higher emissions due to air blowing | Lower emissions with heat recovery |
| Historical Significance | Revolutionized steel production, rapid industrial growth | Allowed higher quality and diverse steel grades |
Introduction to Iron Production Methods
The Bessemer Process revolutionized iron production by enabling the rapid conversion of pig iron into steel through air blowing to remove impurities, significantly reducing manufacturing time and costs. The Open Hearth Process, introduced later, allowed for better control over the steel's composition by using a regenerative furnace to slowly melt pig iron and scrap steel, producing higher-quality steel with fewer defects. Both methods played crucial roles in industrial iron production, with the Bessemer Process emphasizing speed and the Open Hearth Process prioritizing precision and quality.
Overview of the Bessemer Process
The Bessemer Process revolutionized iron production by blowing air through molten pig iron to rapidly oxidize impurities, primarily carbon, reducing production time from days to minutes. This method significantly lowered costs and increased steel output, enabling mass industrialization in the 19th century. Compared to the Open Hearth Process, the Bessemer Process was faster but less flexible in controlling the composition of the final steel product.
Overview of the Open Hearth Process
The Open Hearth Process is a steelmaking method utilizing a regenerative furnace to melt scrap steel and pig iron with controlled air and fuel combustion, allowing precise temperature management and impurity removal. This process offers greater flexibility in raw material input compared to the Bessemer Process, enabling the production of large quantities of high-quality steel with reduced carbon and sulfur content. The Open Hearth Process dominated steel production in the late 19th and early 20th centuries due to its efficiency and ability to produce diverse steel grades.
Historical Significance and Development
The Bessemer Process revolutionized steel production in the 19th century by dramatically reducing costs and increasing output through its rapid, blast-air method, marking a key advancement in the Industrial Revolution. The Open Hearth Process, developed later, enhanced steel quality and allowed greater control over alloy composition, becoming dominant in the early 20th century due to its ability to produce large quantities of more uniform steel. These processes were pivotal in shaping modern infrastructure, transportation, and manufacturing industries by enabling mass production of durable and affordable steel.
Raw Materials and Inputs Comparison
The Bessemer process relies primarily on molten pig iron as its raw material, using air blast to remove impurities rapidly, making it efficient for large-scale steel production. The Open Hearth process utilizes a mixture of pig iron, scrap steel, and iron ore, allowing for more flexible input materials and better control over the final chemical composition. While the Bessemer process requires low phosphorus content in the raw material, the Open Hearth can accommodate higher impurity levels, making it suitable for a wider range of inputs.
Chemical Reactions and Metallurgical Differences
The Bessemer Process rapidly converts molten pig iron into steel by blowing air through the iron to oxidize impurities like carbon, silicon, and manganese, producing slag and releasing carbon dioxide. The Open Hearth Process uses a regenerative furnace allowing precise temperature control and longer reaction times, facilitating the oxidation of carbon and other contaminants through a slower, more controlled reaction with iron oxide and fluxes. Metallurgically, the Bessemer method often results in lower-quality steel due to rapid oxidation and limited impurity control, while the Open Hearth Process yields higher-quality steel with improved homogeneity and lower residual impurities.
Energy Consumption and Efficiency
The Bessemer Process consumes less energy due to its rapid air blast method, which efficiently removes impurities in about 20 minutes, while the Open Hearth Process requires longer heating times, resulting in higher fuel consumption. Despite the lower energy usage, the Open Hearth Process offers greater control over the final chemical composition, enhancing steel quality and reducing waste. Overall, Bessemer is favored for speed and energy efficiency, whereas Open Hearth prioritizes precision and product consistency despite increased energy demands.
Quality and Properties of Produced Iron
The Bessemer Process produces iron with higher carbon content, resulting in harder but more brittle steel, whereas the Open Hearth Process allows for better control of impurities, yielding iron with improved uniformity and ductility. The Open Hearth method enables precise adjustment of carbon and other alloying elements, enhancing tensile strength and toughness compared to Bessemer steel. Consequently, Open Hearth steel is preferred for applications requiring superior quality and consistent mechanical properties.
Environmental Impact of Each Process
The Bessemer Process emits higher levels of carbon dioxide and sulfur compounds due to its rapid oxidation method, contributing significantly to air pollution. The Open Hearth Process, while slower, allows for better control of impurities, resulting in reduced emissions of harmful gases and lower environmental damage. Both processes impact ecosystems through waste slag, but the Open Hearth method's efficiency in refining reduces the overall pollutant output.
Modern Relevance and Industrial Legacy
The Bessemer Process revolutionized steel production by enabling rapid, large-scale manufacturing, laying the foundation for modern industrial steelmaking despite its limitations in controlling impurities. The Open Hearth Process, offering greater control over steel composition and improved quality, dominated throughout the 20th century, influencing contemporary steel refining techniques and standards. Both methods established critical industrial legacies, with principles still evident in current practices emphasizing efficiency, scalability, and material quality in steel production.
Bessemer Process vs Open Hearth Process Infographic
 materialdif.com
materialdif.com