Copper anodizing creates a durable oxide layer on the surface, enhancing corrosion resistance and improving adhesion for subsequent coatings, while copper electroplating involves depositing a thin layer of copper onto a substrate to improve conductivity and aesthetic appeal. Anodizing is typically applied to copper alloys to increase surface hardness and wear resistance, whereas electroplating is favored for applications requiring enhanced electrical conductivity and a uniform metallic finish. Choosing between copper anodizing and electroplating depends on the desired surface properties, with anodizing offering protective benefits and electroplating providing functional and decorative enhancements.
Table of Comparison
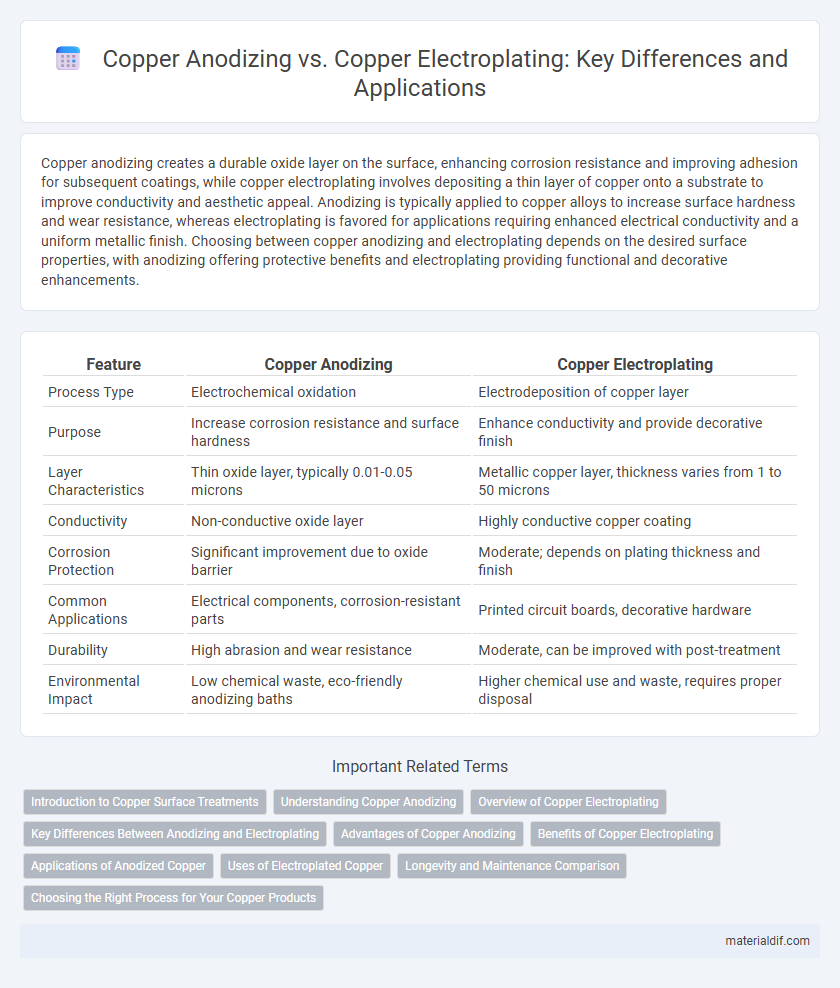
| Feature | Copper Anodizing | Copper Electroplating |
|---|---|---|
| Process Type | Electrochemical oxidation | Electrodeposition of copper layer |
| Purpose | Increase corrosion resistance and surface hardness | Enhance conductivity and provide decorative finish |
| Layer Characteristics | Thin oxide layer, typically 0.01-0.05 microns | Metallic copper layer, thickness varies from 1 to 50 microns |
| Conductivity | Non-conductive oxide layer | Highly conductive copper coating |
| Corrosion Protection | Significant improvement due to oxide barrier | Moderate; depends on plating thickness and finish |
| Common Applications | Electrical components, corrosion-resistant parts | Printed circuit boards, decorative hardware |
| Durability | High abrasion and wear resistance | Moderate, can be improved with post-treatment |
| Environmental Impact | Low chemical waste, eco-friendly anodizing baths | Higher chemical use and waste, requires proper disposal |
Introduction to Copper Surface Treatments
Copper anodizing involves the formation of a protective oxide layer through controlled electrochemical oxidation, enhancing corrosion resistance and surface durability. Copper electroplating deposits a thin layer of copper or another metal onto the surface using an electric current, improving conductivity, appearance, and wear resistance. Both methods serve to extend copper's functional lifespan but differ significantly in process chemistry and the final surface properties achieved.
Understanding Copper Anodizing
Copper anodizing enhances corrosion resistance and surface hardness by forming a controlled oxide layer through electrochemical oxidation, creating a durable protective barrier. This process differs from copper electroplating, which deposits a thin layer of pure copper onto a substrate for decorative or conductive purposes. Understanding anodizing involves recognizing its role in improving copper's wear resistance and insulating properties without significantly altering its appearance.
Overview of Copper Electroplating
Copper electroplating involves depositing a thin layer of copper onto the surface of a metal or plastic substrate through an electrolytic process that enhances conductivity, corrosion resistance, and aesthetic appeal. This technique uses a copper sulfate solution and an electric current to transfer copper ions onto the object, resulting in a uniform, durable coating. The process is widely used in electronics, automotive, and decorative industries for improving surface properties and extending the lifespan of components.
Key Differences Between Anodizing and Electroplating
Copper anodizing creates a durable oxide layer through an electrochemical process that enhances corrosion resistance and surface hardness without adding a metal coating. Copper electroplating deposits a thin layer of copper onto a substrate using an electrical current, improving conductivity and aesthetic appeal while also providing some corrosion protection. The primary difference lies in anodizing forming an integral oxide layer on the copper itself, whereas electroplating applies an external copper coating onto another metal.
Advantages of Copper Anodizing
Copper anodizing enhances corrosion resistance and surface hardness, making it ideal for industrial applications requiring durable and wear-resistant coatings. This process produces a consistent, environmentally friendly oxide layer that improves electrical insulation and adhesion for subsequent coatings. The anodized surface also offers superior aesthetic appeal with customizable colors without the risk of peeling or flaking common in electroplated copper.
Benefits of Copper Electroplating
Copper electroplating offers enhanced corrosion resistance by forming a uniform, dense metallic layer that protects the base material from environmental damage. This process improves electrical conductivity and wear resistance, making it ideal for electronic components and industrial applications. Unlike copper anodizing, electroplating provides superior adhesion and a more aesthetically consistent finish suitable for decorative purposes.
Applications of Anodized Copper
Anodized copper is commonly employed in architectural applications, where its enhanced corrosion resistance and appealing patina improve both durability and aesthetics in exterior cladding and decorative elements. It is also used in electronic components, such as printed circuit boards (PCBs), where the anodized layer provides superior insulation and protection against oxidation. Industrial machinery benefits from anodized copper parts due to their increased hardness and wear resistance, extending the lifespan of critical components.
Uses of Electroplated Copper
Electroplated copper is widely used in electronics manufacturing for printed circuit boards (PCBs) due to its excellent electrical conductivity and corrosion resistance. It provides a smooth, uniform coating that enhances solderability and ensures reliable electrical connections in microelectronics and connectors. This technique is also applied in decorative finishes and improving wear resistance in various industrial components.
Longevity and Maintenance Comparison
Copper anodizing significantly enhances surface hardness and corrosion resistance, resulting in longer-lasting protection compared to copper electroplating, which primarily adds a decorative layer prone to wear. Maintenance of anodized copper requires less frequent intervention due to its durable oxide layer, whereas electroplated copper demands regular upkeep to prevent tarnishing and peeling. The superior longevity and minimal maintenance needs of anodized copper make it a preferred choice for industrial applications exposed to harsh environments.
Choosing the Right Process for Your Copper Products
Copper anodizing enhances corrosion resistance and surface hardness by forming a controlled oxide layer, ideal for applications requiring durability and electrical insulation. Copper electroplating deposits a thin, pure copper layer onto a substrate, improving conductivity and aesthetic appeal, suitable for decorative and electronic components. Selecting the right process depends on product requirements: anodizing suits wear resistance and insulation, while electroplating excels in enhancing conductivity and appearance.
Copper Anodizing vs Copper Electroplating Infographic
 materialdif.com
materialdif.com