Natural graphite, sourced directly from mined deposits, offers a cost-effective and eco-friendly option due to its minimal processing requirements, making it suitable for applications prioritizing sustainability. Synthetic graphite, produced through high-temperature treatment of carbon-rich precursors, provides superior purity, uniformity, and enhanced electrochemical properties essential for high-performance lithium-ion batteries in electric vehicles. The choice between natural and synthetic graphite depends on balancing factors such as cost, performance demands, and environmental impact within carbon PET production.
Table of Comparison
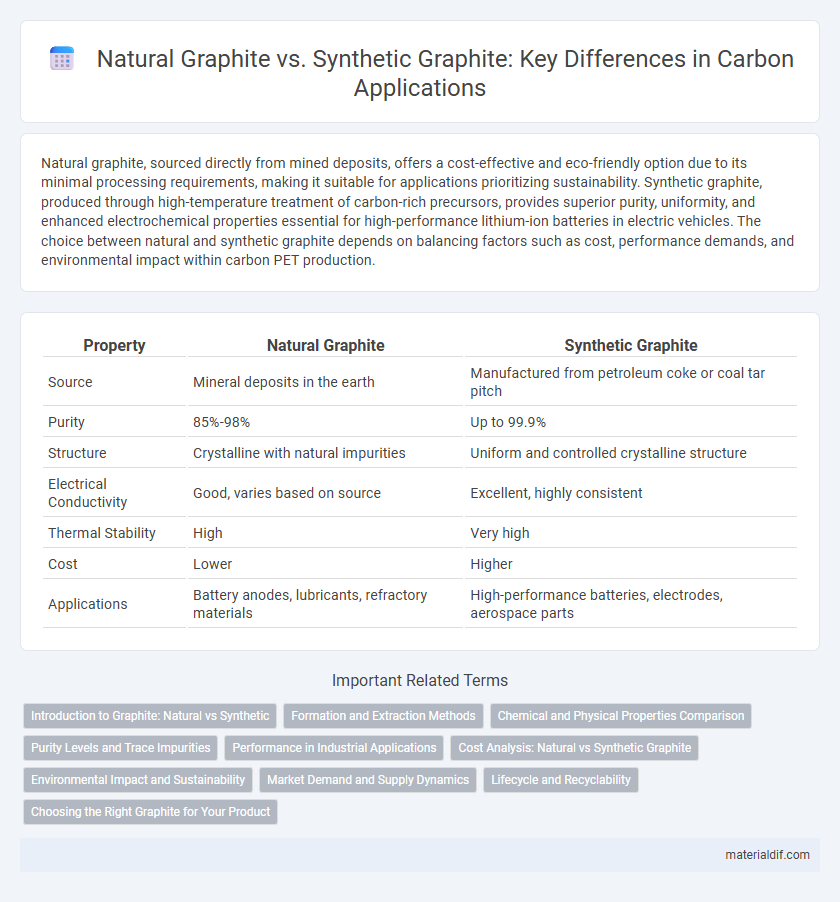
| Property | Natural Graphite | Synthetic Graphite |
|---|---|---|
| Source | Mineral deposits in the earth | Manufactured from petroleum coke or coal tar pitch |
| Purity | 85%-98% | Up to 99.9% |
| Structure | Crystalline with natural impurities | Uniform and controlled crystalline structure |
| Electrical Conductivity | Good, varies based on source | Excellent, highly consistent |
| Thermal Stability | High | Very high |
| Cost | Lower | Higher |
| Applications | Battery anodes, lubricants, refractory materials | High-performance batteries, electrodes, aerospace parts |
Introduction to Graphite: Natural vs Synthetic
Natural graphite is a naturally occurring form of crystalline carbon with a layered structure, prized for its high conductivity and lubricating properties. Synthetic graphite, manufactured through high-temperature treatment of carbon-rich precursors like petroleum coke, offers consistent purity and tailored physical characteristics. Applications in batteries, refractories, and electrodes benefit from the distinct advantages of each graphite type, driven by factors such as particle size, impurity levels, and structural uniformity.
Formation and Extraction Methods
Natural graphite forms through high-temperature metamorphic processes in carbon-rich sedimentary rocks, resulting in crystalline structures extracted via open-pit or underground mining. Synthetic graphite is produced by heating petroleum coke and coal tar pitch at temperatures above 2500degC in electric furnaces, followed by shaping and purification methods. The extraction of natural graphite involves beneficiation techniques like flotation, while synthetic graphite requires controlled thermal treatment to achieve desired purity and structural properties.
Chemical and Physical Properties Comparison
Natural graphite exhibits a crystalline structure with impurities like silicates, affecting its purity and electrical conductivity, whereas synthetic graphite is highly pure with controlled particle size and fewer defects. Chemically, synthetic graphite shows greater consistency in carbon content, often exceeding 99.9%, while natural graphite varies between 80% to 98% carbon depending on the deposit. Physically, synthetic graphite has higher density and superior thermal stability, making it ideal for advanced industrial applications, while natural graphite offers better lubricity and flexibility.
Purity Levels and Trace Impurities
Natural graphite typically contains purity levels ranging from 85% to 95%, with trace impurities such as quartz, mica, and feldspar that can affect its performance in high-purity applications. Synthetic graphite offers higher purity, often exceeding 99.9%, with minimal trace impurities due to controlled manufacturing processes, making it suitable for advanced industries like lithium-ion batteries and electronics. The choice between natural and synthetic graphite depends on the required purity levels and tolerance for specific trace contaminants in the final product.
Performance in Industrial Applications
Natural graphite offers superior thermal conductivity and cost-efficiency in industrial applications such as lubricants and batteries, while synthetic graphite provides enhanced purity, uniform particle size, and mechanical strength, making it ideal for high-performance uses in electrodes and refractory materials. Synthetic graphite's controlled microstructure results in better consistency and reliability in demanding environments, whereas natural graphite excels in applications requiring more conductive and flexible materials. Both materials contribute uniquely to industrial efficiency, with synthetic graphite favored for precision and durability and natural graphite preferred for cost-sensitive, large-scale uses.
Cost Analysis: Natural vs Synthetic Graphite
Natural graphite typically incurs lower production costs due to its abundant availability and less energy-intensive extraction processes, making it more cost-effective for large-scale industrial applications. Synthetic graphite, derived from carbon-rich precursors like petroleum coke through high-temperature treatment, has higher manufacturing expenses driven by energy consumption and complex processing techniques. Despite higher initial costs, synthetic graphite offers superior purity and consistency, which can justify investments in specialized sectors such as battery anodes and advanced electronics.
Environmental Impact and Sustainability
Natural graphite mining involves significant land disruption and water consumption, leading to habitat loss and pollution, while synthetic graphite production relies heavily on fossil fuels, resulting in higher carbon emissions. Despite synthetic graphite's energy-intensive manufacturing, it offers more consistent quality and potential for recycling, which can reduce overall environmental impact. Sustainable graphite sourcing increasingly prioritizes recycled materials and cleaner production technologies to minimize ecological footprints.
Market Demand and Supply Dynamics
Natural graphite, sourced from mining operations, faces supply constraints due to limited high-quality deposits and environmental regulations, driving up demand for synthetic graphite produced through controlled manufacturing processes. Synthetic graphite, while more costly to produce, offers consistent quality and purity, making it increasingly favored in high-tech applications like lithium-ion batteries, which fuels market growth. Shifts in energy storage and electric vehicle industries are intensifying demand for synthetic graphite, balancing the overall market dynamics between natural availability and engineered supply.
Lifecycle and Recyclability
Natural graphite, derived from mining, offers a lower environmental impact during extraction but tends to have a longer and less energy-intensive lifecycle compared to synthetic graphite, which is produced through high-temperature processes consuming significant energy. Synthetic graphite provides uniform quality and higher purity but poses challenges in recyclability due to complex manufacturing residues and bonding agents. Efficient recycling systems for natural graphite, involving mechanical and chemical treatments, enhance material recovery and reduce environmental footprint throughout its lifecycle.
Choosing the Right Graphite for Your Product
Natural graphite offers high purity and excellent thermal conductivity, making it ideal for applications requiring superior electrical performance and heat resistance. Synthetic graphite provides consistent particle size and shape with customizable properties, suitable for high-precision industries such as aerospace and battery manufacturing. Evaluating factors like cost, performance requirements, and production scalability ensures the optimal graphite type aligns with your product's functionality and budget constraints.
Natural Graphite vs Synthetic Graphite Infographic
 materialdif.com
materialdif.com