Cladding involves bonding a thick layer of one metal onto another, providing enhanced durability and corrosion resistance compared to plating, which applies a thinner metallic coating through electrochemical processes. Cladding offers superior wear resistance and longevity, making it ideal for high-stress applications, while plating is suited for decorative finishes and moderate protection. Choosing between cladding and plating depends on the desired performance, cost, and environmental exposure of the alloy pet product.
Table of Comparison
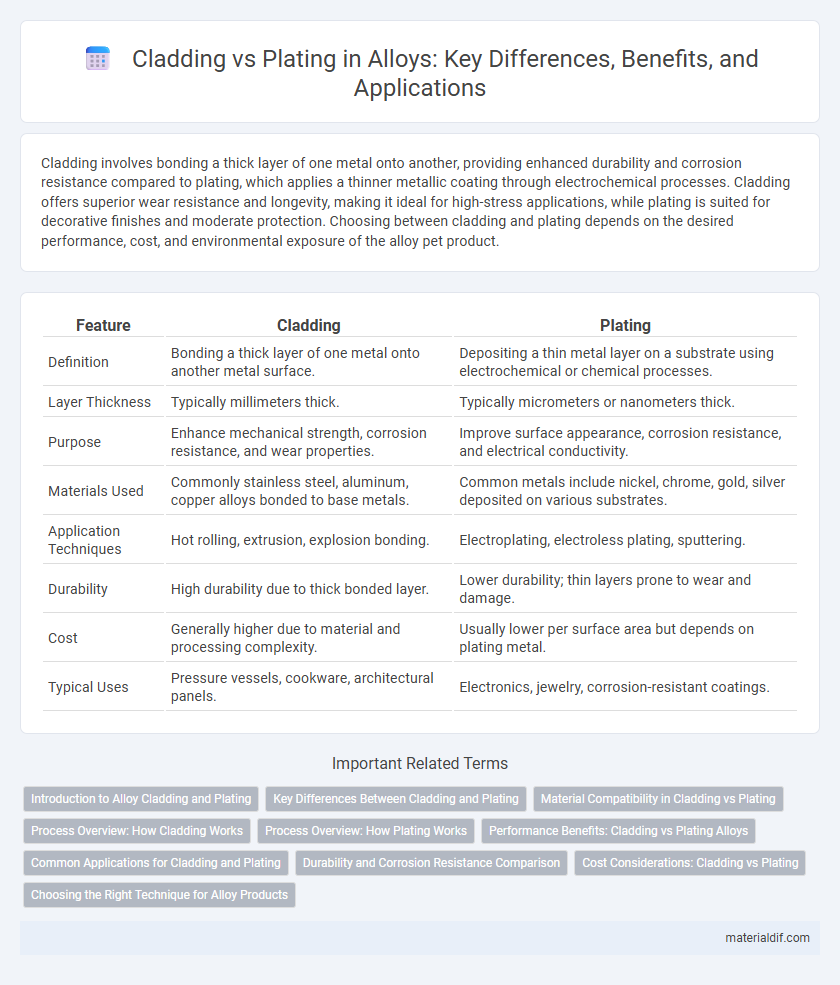
| Feature | Cladding | Plating |
|---|---|---|
| Definition | Bonding a thick layer of one metal onto another metal surface. | Depositing a thin metal layer on a substrate using electrochemical or chemical processes. |
| Layer Thickness | Typically millimeters thick. | Typically micrometers or nanometers thick. |
| Purpose | Enhance mechanical strength, corrosion resistance, and wear properties. | Improve surface appearance, corrosion resistance, and electrical conductivity. |
| Materials Used | Commonly stainless steel, aluminum, copper alloys bonded to base metals. | Common metals include nickel, chrome, gold, silver deposited on various substrates. |
| Application Techniques | Hot rolling, extrusion, explosion bonding. | Electroplating, electroless plating, sputtering. |
| Durability | High durability due to thick bonded layer. | Lower durability; thin layers prone to wear and damage. |
| Cost | Generally higher due to material and processing complexity. | Usually lower per surface area but depends on plating metal. |
| Typical Uses | Pressure vessels, cookware, architectural panels. | Electronics, jewelry, corrosion-resistant coatings. |
Introduction to Alloy Cladding and Plating
Alloy cladding involves bonding a layer of corrosion-resistant or wear-resistant metal onto a base alloy to enhance surface properties without compromising the core material. Plating, on the other hand, deposits a thin metallic layer through electrochemical or electroplating processes, providing surface protection and aesthetic appeal. Both cladding and plating improve alloy durability, with cladding offering thicker, more robust layers and plating allowing precise control over coating thickness.
Key Differences Between Cladding and Plating
Cladding involves bonding a layer of one metal onto the surface of another to enhance corrosion resistance and mechanical properties, while plating deposits a thin metal coating via electrochemical or chemical processes primarily for aesthetic or protective purposes. Cladding produces a thicker, metallurgically bonded layer, offering superior durability compared to the generally thinner, surface-level plating. Key differences include the thickness of the applied layer, bonding method, and resulting mechanical strength and corrosion resistance.
Material Compatibility in Cladding vs Plating
Material compatibility in cladding ensures the base metal and cladding layer bond metallurgically, providing uniform corrosion resistance and mechanical properties, often involving similar alloys to prevent galvanic corrosion. In plating, surface compatibility depends on proper adhesion and intermediate layers, with dissimilar materials requiring careful selection to avoid peeling or electrochemical degradation. Cladding typically offers superior structural integration, while plating emphasizes surface-level protection tailored by compatibility between substrate and plating metal.
Process Overview: How Cladding Works
Cladding involves bonding two or more layers of metals by applying heat and pressure, creating a composite material with enhanced properties such as corrosion resistance and strength. Unlike plating, which deposits a thin metallic layer via electrochemical or mechanical means, cladding fuses materials at a metallurgical level, ensuring greater durability and homogeneity. This process typically uses techniques like roll bonding, explosive welding, or diffusion bonding to achieve a seamless and robust composite alloy.
Process Overview: How Plating Works
Plating involves depositing a thin layer of metal onto a substrate through electrochemical or electroless techniques, creating a protective or decorative coating. In electroplating, an electric current drives metal ions from a solution to the substrate surface, ensuring uniform coverage and enhanced corrosion resistance. This process differs from cladding, which mechanically bonds thicker metal layers, offering structural integrity along with surface protection.
Performance Benefits: Cladding vs Plating Alloys
Cladding alloys offer superior corrosion resistance and enhanced mechanical strength by bonding dissimilar metals, creating a composite structure that leverages the best properties of each layer. Plating alloys typically provide a thinner, more uniform protective coating that improves surface hardness and wear resistance but may lack the structural robustness of cladding. Performance benefits of cladding include greater durability in harsh environments and improved thermal conductivity, while plating is often chosen for aesthetic finishes and targeted surface protection.
Common Applications for Cladding and Plating
Cladding is commonly used in architectural facades, aerospace components, and cookware due to its ability to bond different metals, enhancing corrosion resistance and mechanical properties without substantial material cost increase. Plating finds widespread applications in electronics for improved conductivity, automotive parts for wear resistance, and jewelry to provide aesthetic finishes and oxidation protection. Both techniques serve critical roles in industries requiring surface enhancement and material optimization.
Durability and Corrosion Resistance Comparison
Cladding offers superior durability by bonding a corrosion-resistant alloy layer to a strong base metal, providing long-lasting protection against wear and environmental damage. Plating, while effective for corrosion resistance, tends to be thinner and more prone to wear or damage over time, requiring more frequent maintenance or reapplication. The metallurgical bond in cladding enhances resistance to corrosion and mechanical stress, making it ideal for demanding industrial applications.
Cost Considerations: Cladding vs Plating
Cladding generally incurs higher initial costs due to thicker material layers and more complex bonding processes, making it more suitable for applications requiring enhanced durability and corrosion resistance. Plating offers a cost-effective alternative with thinner metal coatings, reducing material usage and processing time but often requiring more frequent maintenance or replacement. Cost efficiency depends on the specific alloy materials, desired surface properties, and long-term performance requirements in industrial applications.
Choosing the Right Technique for Alloy Products
Cladding and plating are essential surface engineering techniques used to enhance alloy products, each offering distinct benefits based on application requirements. Cladding involves bonding a thicker layer of one metal over another to improve corrosion resistance and mechanical strength, making it ideal for high-stress environments, while plating applies a thinner metal coating primarily for aesthetic appeal and surface protection. Selecting the right technique depends on factors such as desired durability, cost efficiency, and environmental exposure, with cladding suited for heavy-duty uses and plating preferred for lightweight corrosion resistance and decorative finishes.
Cladding vs Plating Infographic
 materialdif.com
materialdif.com