Medical grade plastic is engineered to meet stringent biocompatibility and sterilization standards, ensuring safety for use in surgical instruments, implants, and medical devices. Food grade plastic is designed to prevent contamination and chemical leaching, making it suitable for packaging and storing consumables. While both types prioritize safety, medical grade plastic undergoes more rigorous testing for toxicity and durability under sterile conditions.
Table of Comparison
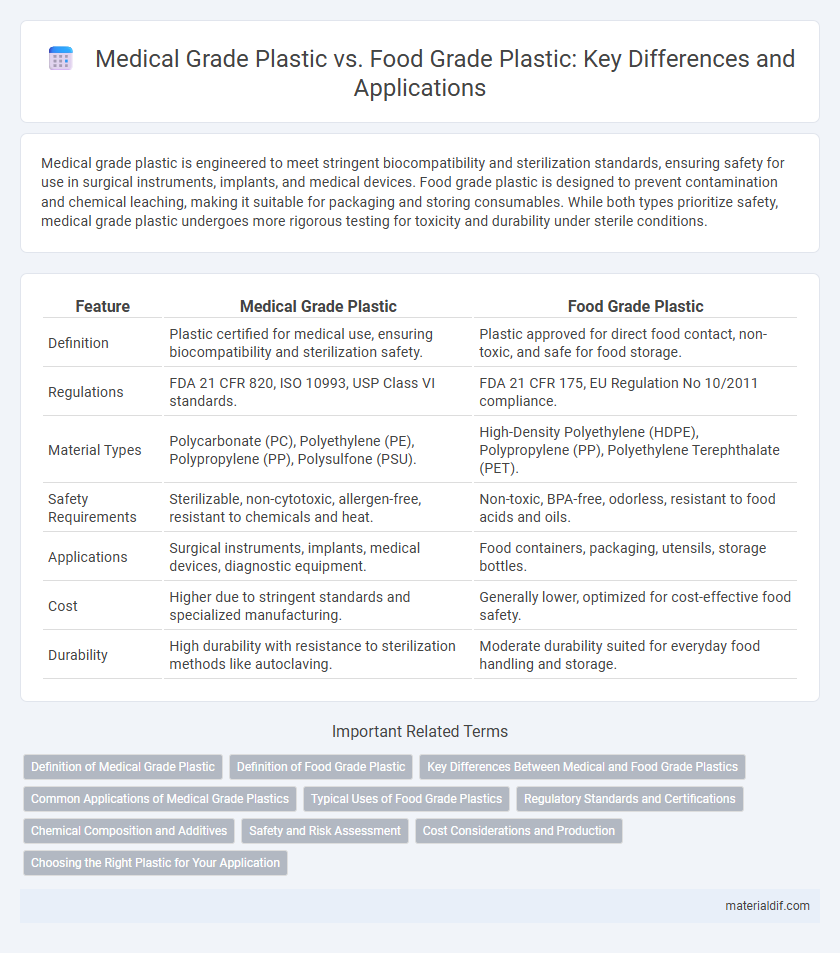
| Feature | Medical Grade Plastic | Food Grade Plastic |
|---|---|---|
| Definition | Plastic certified for medical use, ensuring biocompatibility and sterilization safety. | Plastic approved for direct food contact, non-toxic, and safe for food storage. |
| Regulations | FDA 21 CFR 820, ISO 10993, USP Class VI standards. | FDA 21 CFR 175, EU Regulation No 10/2011 compliance. |
| Material Types | Polycarbonate (PC), Polyethylene (PE), Polypropylene (PP), Polysulfone (PSU). | High-Density Polyethylene (HDPE), Polypropylene (PP), Polyethylene Terephthalate (PET). |
| Safety Requirements | Sterilizable, non-cytotoxic, allergen-free, resistant to chemicals and heat. | Non-toxic, BPA-free, odorless, resistant to food acids and oils. |
| Applications | Surgical instruments, implants, medical devices, diagnostic equipment. | Food containers, packaging, utensils, storage bottles. |
| Cost | Higher due to stringent standards and specialized manufacturing. | Generally lower, optimized for cost-effective food safety. |
| Durability | High durability with resistance to sterilization methods like autoclaving. | Moderate durability suited for everyday food handling and storage. |
Definition of Medical Grade Plastic
Medical grade plastic refers to high-purity polymer materials specifically engineered to comply with stringent biocompatibility standards and sterilization requirements for use in medical devices, implants, and packaging. These plastics undergo rigorous testing for toxicity, chemical resistance, and mechanical performance to ensure patient safety and regulatory compliance, such as FDA or ISO certifications. Unlike food grade plastic, which is designed primarily to prevent contamination and maintain food quality, medical grade variants emphasize sterility, durability, and non-reactivity in clinical environments.
Definition of Food Grade Plastic
Food grade plastic is defined by its compliance with strict safety standards set by regulatory agencies such as the FDA, ensuring it is free from harmful chemicals and safe for contact with food and beverages. This type of plastic resists leaching and contamination, maintaining the purity and integrity of consumables stored or processed within it. Common examples include polyethylene and polypropylene, widely used in packaging, containers, and utensils designed for food storage and handling.
Key Differences Between Medical and Food Grade Plastics
Medical grade plastics are designed to meet stringent biocompatibility and sterilization standards, ensuring safety for direct contact with human tissues and fluids. Food grade plastics comply with regulations that prevent contamination and maintain food safety, focusing on chemical resistance and non-toxicity for consumption purposes. Key differences include the level of purity, regulatory requirements such as FDA or ISO certifications, and the types of additives allowed, which vary based on the intended application and exposure risks.
Common Applications of Medical Grade Plastics
Medical grade plastics are commonly used in applications requiring high biocompatibility and sterilization capability, such as surgical instruments, intravenous tubing, and implantable devices. These plastics must meet stringent regulatory standards set by agencies like the FDA or ISO for use in healthcare environments. Their chemical resistance and durability also make them ideal for diagnostic equipment and protective barriers in clinical settings.
Typical Uses of Food Grade Plastics
Food grade plastics are commonly used in packaging materials such as containers, bottles, and wraps designed to store and preserve consumables safely. These plastics meet strict regulatory standards to prevent contamination and are frequently found in kitchen utensils, cutting boards, and food processing equipment. Their non-toxic properties ensure they do not leach harmful chemicals into food products during storage or preparation.
Regulatory Standards and Certifications
Medical grade plastic complies with stringent regulatory standards such as ISO 10993 and FDA CFR 21, ensuring biocompatibility and safety for use in medical devices and implants. Food grade plastic meets regulations like FDA 21 CFR 177 and EU 10/2011, guaranteeing that materials are safe for contact with food without leaching harmful substances. Certifications such as NSF and USP Class VI validate the safety and quality of these plastics within their respective applications, emphasizing their adherence to health and safety requirements.
Chemical Composition and Additives
Medical grade plastic is engineered with stringent chemical compositions that prioritize biocompatibility and sterilization resistance, often containing minimal additives to prevent toxicity and allergic reactions. Food grade plastic incorporates specific additives approved for food contact, such as stabilizers and plasticizers, designed to prevent chemical migration and ensure safety during storage and consumption. Both types require compliance with regulatory standards, but medical grade plastics typically undergo stricter testing for chemical purity and additive safety to meet healthcare application demands.
Safety and Risk Assessment
Medical grade plastic undergoes rigorous sterilization and biocompatibility testing to ensure safety for direct contact with human tissues, minimizing risks of toxicity and contamination. Food grade plastic is regulated to prevent chemical leaching and maintain food safety but lacks the stringent requirements for prolonged or invasive human exposure found in medical applications. Risk assessment for medical grade plastics emphasizes sterility, endotoxin levels, and cytotoxicity, while food grade plastic assessments focus primarily on migration limits of harmful substances into food products.
Cost Considerations and Production
Medical grade plastic requires rigorous testing, higher purity standards, and specialized additives, leading to significantly higher production costs compared to food grade plastic. Food grade plastic production focuses on safety for consumption with less stringent certifications, making it more cost-effective for mass manufacturing. The complex processing and regulatory compliance in medical grade plastics increase overall expenses and limit scalability in contrast to food grade materials.
Choosing the Right Plastic for Your Application
Medical grade plastic undergoes rigorous testing for biocompatibility, sterilization resistance, and chemical inertness, making it ideal for healthcare devices and implants where safety and hygiene are critical. Food grade plastic complies with FDA and EU regulations for food contact, ensuring it does not leach harmful substances into consumables, suitable for packaging and storage containers. Selecting the right plastic depends on the application's exposure to chemicals, temperature, and regulatory requirements to ensure safety and performance.
Medical Grade Plastic vs Food Grade Plastic Infographic
 materialdif.com
materialdif.com