Sulfamate nickel plating provides superior brightness, ductility, and corrosion resistance compared to Watts nickel, making it ideal for decorative and functional coatings. Unlike Watts nickel, which typically uses a nickel sulfate and chloride bath resulting in higher internal stress, sulfamate nickel baths produce low-stress deposits that enhance durability and reduce cracking. This makes sulfamate nickel a preferred choice for precision components and industries requiring high-performance nickel finishes.
Table of Comparison
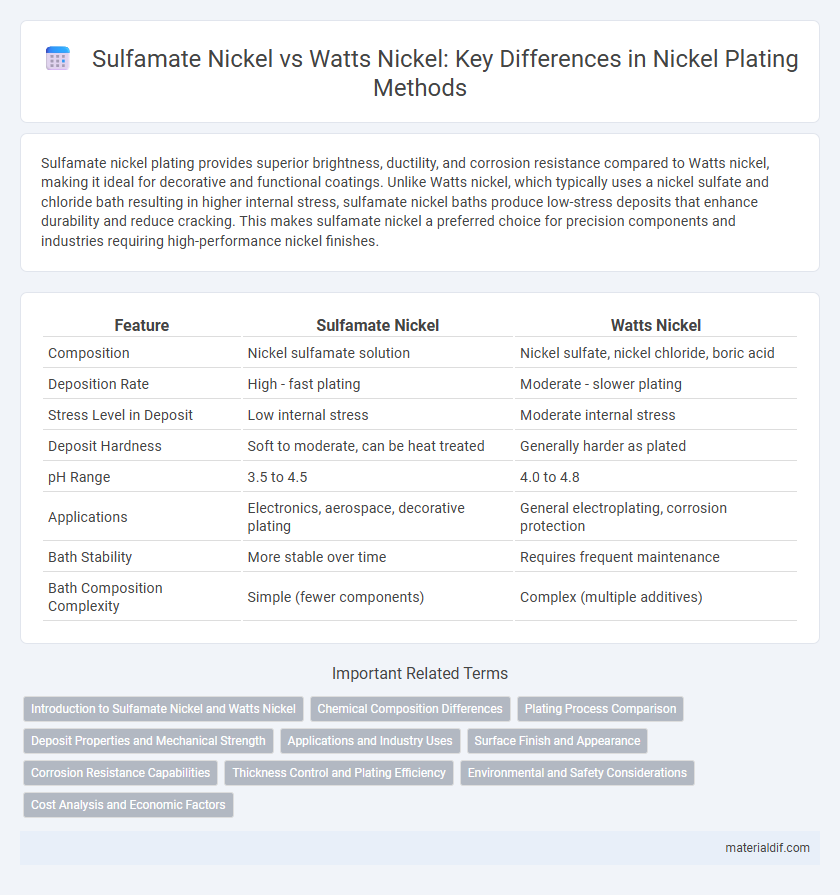
| Feature | Sulfamate Nickel | Watts Nickel |
|---|---|---|
| Composition | Nickel sulfamate solution | Nickel sulfate, nickel chloride, boric acid |
| Deposition Rate | High - fast plating | Moderate - slower plating |
| Stress Level in Deposit | Low internal stress | Moderate internal stress |
| Deposit Hardness | Soft to moderate, can be heat treated | Generally harder as plated |
| pH Range | 3.5 to 4.5 | 4.0 to 4.8 |
| Applications | Electronics, aerospace, decorative plating | General electroplating, corrosion protection |
| Bath Stability | More stable over time | Requires frequent maintenance |
| Bath Composition Complexity | Simple (fewer components) | Complex (multiple additives) |
Introduction to Sulfamate Nickel and Watts Nickel
Sulfamate nickel plating offers superior throwing power and low internal stress, making it ideal for complex shapes and applications requiring high corrosion resistance. Watts nickel, composed primarily of nickel sulfate, nickel chloride, and boric acid, is widely used for general-purpose plating with good brightness and uniformity. Both electrolytic solutions serve different industrial needs, with sulfamate nickel favoring structural integrity and Watts nickel excelling in decorative finishes.
Chemical Composition Differences
Sulfamate nickel plating solutions primarily consist of nickel sulfamate (Ni(NH2SO3)2), which provides high-purity nickel deposits with excellent ductility and low internal stress. Watts nickel baths use nickel sulfate (NiSO4), nickel chloride (NiCl2), and boric acid, resulting in harder deposits with higher tensile strength but increased internal stress compared to sulfamate nickel. The chemical composition differences influence the plating applications, with sulfamate suitable for electroforming and precision plating, while Watts is preferred for wear-resistant coatings.
Plating Process Comparison
Sulfamate nickel plating offers superior thickness control and lower internal stress compared to Watts nickel plating, making it ideal for applications requiring precise and durable coatings. Watts nickel plating utilizes a nickel sulfate bath with added nickel chloride and boric acid, providing good brightness but typically higher internal stress and less ductility than sulfamate baths. The sulfamate process allows for higher plating rates and better hydrogen embrittlement resistance, enhancing its suitability for aerospace and electronics industries.
Deposit Properties and Mechanical Strength
Sulfamate nickel deposits exhibit exceptional ductility and low internal stress, which results in smoother, more uniform coatings ideal for precision plating applications. Watts nickel deposits tend to have higher hardness and tensile strength but are more prone to brittleness and internal stress, making them suitable for wear-resistant surfaces. The superior mechanical strength of Watts nickel complements its robustness, while sulfamate nickel's superior deposit properties favor flexibility and corrosion resistance in demanding environments.
Applications and Industry Uses
Sulfamate nickel plating offers superior stress relief and exceptional brightness, making it ideal for precision electronic components, aerospace parts, and automotive applications requiring high ductility and corrosion resistance. Watts nickel plating, known for its high hardness and wear resistance, is widely used in industrial machinery, tooling, and chemical equipment where durability and protection against abrasive environments are critical. Both types are integral in the electronics, aerospace, automotive, and manufacturing sectors, with Sulfamate nickel favored for specialized high-performance coatings and Watts nickel for robust, heavy-duty industrial applications.
Surface Finish and Appearance
Sulfamate nickel plating provides a smoother, more ductile surface finish compared to Watts nickel, making it ideal for decorative and precision applications requiring a flawless appearance. Its ability to deposit thick, stress-free coatings enhances corrosion resistance and produces a bright, uniform layer that resists cracking and pitting. Watts nickel plating, while cost-effective, often results in a coarser surface with higher internal stress, which can lead to duller finishes and less consistent aesthetics.
Corrosion Resistance Capabilities
Sulfamate nickel plating offers superior corrosion resistance compared to Watts nickel due to its denser and more uniform deposit, which reduces porosity and enhances protection against harsh environments. The sulfamate bath produces low internal stress coatings that resist cracking and pitting, making it ideal for aerospace and marine applications. Watts nickel plating, while cost-effective, typically exhibits higher porosity, leading to greater susceptibility to corrosion under prolonged exposure to saltwater or acidic conditions.
Thickness Control and Plating Efficiency
Sulfamate nickel plating offers superior thickness control due to its stable bath chemistry, enabling precise deposition rates critical for uniform coatings. Watts nickel, while widely used, often exhibits less consistent thickness uniformity because of its electrolyte composition, leading to potential over-plating in complex geometries. Plating efficiency is higher in sulfamate nickel baths, resulting in reduced energy consumption and lower waste generation compared to Watts nickel processes.
Environmental and Safety Considerations
Sulfamate nickel plating offers environmental advantages over Watts nickel by producing less hazardous waste due to its lower content of nickel sulfate and chloride, reducing the risk of soil and water contamination. Sulfamate baths operate at lower temperatures and pH levels, minimizing energy consumption and chemical hazards, which enhances workplace safety. Watts nickel plating involves higher concentrations of nickel sulfate and acid, increasing the potential for toxic fumes and demanding more stringent handling and disposal protocols to protect workers and the environment.
Cost Analysis and Economic Factors
Sulfamate nickel plating often incurs higher initial costs due to the price of sulfamate nickel salts and more specialized bath maintenance requirements compared to Watts nickel, which uses more readily available nickel sulfate and nickel chloride. Economic factors also include the increased deposition efficiency and lower internal stress of sulfamate nickel, potentially reducing rework and extending component lifespan, thereby offering long-term cost savings. Watts nickel baths demand more frequent replenishment and generate waste with higher nickel content, increasing operational and environmental compliance costs relative to sulfamate nickel processes.
Sulfamate Nickel vs Watts Nickel Infographic
 materialdif.com
materialdif.com