Nickel sulfate and nickel chloride are both commonly used nickel salts in electroplating and chemical industries, with nickel sulfate favored for its lower corrosion potential and more stable crystalline structure. Nickel chloride offers higher solubility and better conductivity, making it ideal for bright nickel plating applications. Selecting the appropriate compound depends on specific process requirements, including bath composition, operating conditions, and desired coating properties.
Table of Comparison
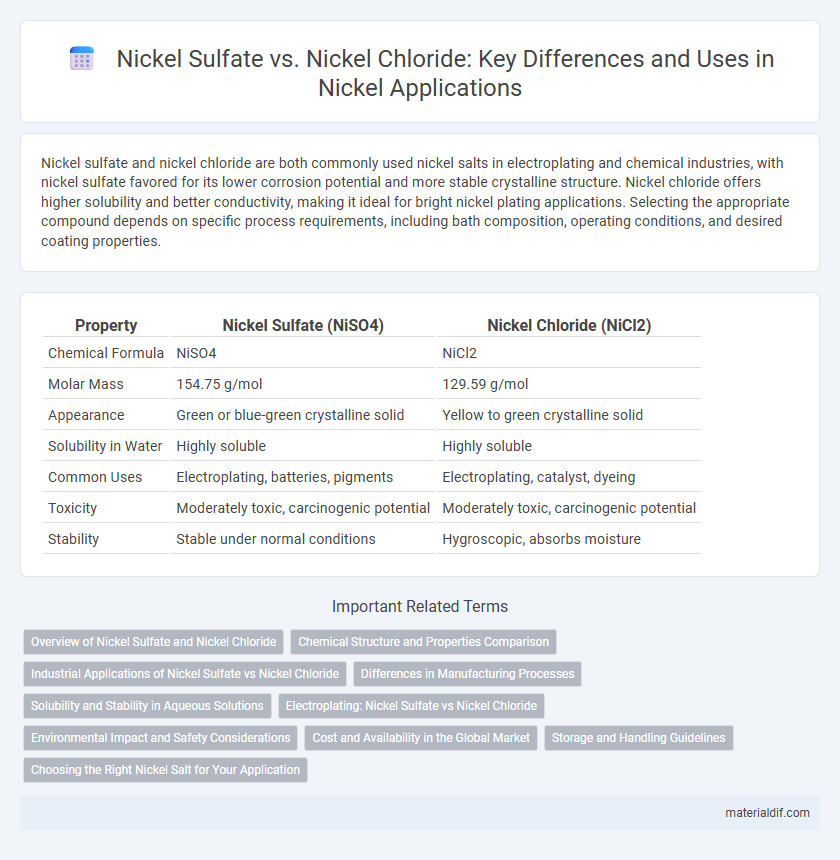
| Property | Nickel Sulfate (NiSO4) | Nickel Chloride (NiCl2) |
|---|---|---|
| Chemical Formula | NiSO4 | NiCl2 |
| Molar Mass | 154.75 g/mol | 129.59 g/mol |
| Appearance | Green or blue-green crystalline solid | Yellow to green crystalline solid |
| Solubility in Water | Highly soluble | Highly soluble |
| Common Uses | Electroplating, batteries, pigments | Electroplating, catalyst, dyeing |
| Toxicity | Moderately toxic, carcinogenic potential | Moderately toxic, carcinogenic potential |
| Stability | Stable under normal conditions | Hygroscopic, absorbs moisture |
Overview of Nickel Sulfate and Nickel Chloride
Nickel sulfate is a green crystalline compound primarily used in electroplating and battery production, offering high solubility and stability. Nickel chloride, typically found as a yellow-brown hexahydrate, is widely employed in catalysis and as a precursor for chemical synthesis due to its strong Lewis acid properties. Both compounds serve as essential nickel salts but differ significantly in their chemical behavior and industrial applications.
Chemical Structure and Properties Comparison
Nickel sulfate (NiSO4) consists of nickel ions complexed with sulfate anions, forming a hexahydrate crystalline structure that exhibits high solubility in water and stable oxidation states. In contrast, nickel chloride (NiCl2) features nickel ions bonded to chloride ions, typically forming dihydrate crystals with distinctive magnetic and color properties due to varying coordination geometry. The chemical structure of nickel sulfate promotes stronger ionic bonding and higher hydration states, while nickel chloride's structure favors more prominent ligand exchange and catalytic activity in industrial processes.
Industrial Applications of Nickel Sulfate vs Nickel Chloride
Nickel sulfate is primarily utilized in electroplating industries for producing corrosion-resistant coatings, while nickel chloride is more commonly used as a catalyst in chemical synthesis and in battery manufacturing. Nickel sulfate's high solubility and stability make it ideal for electrodeposition processes, whereas nickel chloride's reactivity supports applications in fuel cells and hybrid battery technologies. Industrial sectors leverage nickel sulfate for producing stainless steel and electronics, while nickel chloride contributes to specialty chemical production and energy storage solutions.
Differences in Manufacturing Processes
Nickel sulfate is primarily produced through the atmospheric or pressure leaching of nickel-containing ores, followed by purification and crystallization, whereas nickel chloride is commonly manufactured via the direct chlorination of nickel metal or refining of air-scrap nickel alloys. The production of nickel sulfate relies heavily on hydrometallurgical techniques involving sulfuric acid, while nickel chloride production involves more complex handling of chlorine gas and hydrochloric acid, resulting in different impurities and physical properties. These distinct manufacturing processes affect the end-use applications, with nickel sulfate favored in battery cathode precursors and nickel chloride commonly used in electroplating and catalyst preparation.
Solubility and Stability in Aqueous Solutions
Nickel sulfate exhibits high solubility in water, forming stable aqueous solutions commonly used in electroplating and battery applications. In contrast, nickel chloride, while also soluble, tends to hydrolyze more readily, resulting in less stable solutions under neutral to basic pH conditions. The enhanced stability of nickel sulfate solutions is attributed to its sulfate anion, which stabilizes nickel ions and minimizes precipitation compared to the chloride counterpart.
Electroplating: Nickel Sulfate vs Nickel Chloride
Nickel sulfate and nickel chloride are essential electrolytes in electroplating, with nickel sulfate providing stable nickel ions for uniform and smoother deposits. Nickel chloride enhances anode corrosion, improving plating consistency but can increase bath acidity, requiring careful pH control. Electroplating formulations typically balance nickel sulfate's conductivity and chloride's activation to optimize coating quality and efficiency.
Environmental Impact and Safety Considerations
Nickel sulfate and nickel chloride differ significantly in their environmental impact and safety considerations; nickel chloride is more corrosive and poses a higher risk of respiratory and skin irritation, requiring stricter handling protocols. Both compounds can contaminate water sources, but nickel sulfate is generally considered less hazardous due to its lower solubility and slower release of nickel ions, reducing toxicity to aquatic life. Proper disposal and containment measures are essential for both to minimize soil and water contamination, with nickel chloride demanding heightened precautions due to its greater environmental persistence and toxicity.
Cost and Availability in the Global Market
Nickel sulfate generally commands a higher price than nickel chloride due to its widespread use in battery manufacturing, driving significant demand in the electric vehicle sector. Nickel chloride is more readily available and often sourced from primary nickel producers, making it a cost-effective option for electroplating and chemical synthesis applications. Global market fluctuations in nickel raw material extraction heavily impact the pricing and availability of both compounds, with supply chain dynamics influenced by mining outputs and refining capacities in major producing countries like Indonesia and the Philippines.
Storage and Handling Guidelines
Nickel sulfate and nickel chloride require storage in tightly sealed containers to prevent moisture absorption and contamination, as both are highly hygroscopic. They should be kept in cool, dry, and well-ventilated areas, away from incompatible substances such as strong acids and oxidizing agents. Proper handling includes using personal protective equipment like gloves and masks to avoid skin contact and inhalation due to their toxic and irritant properties.
Choosing the Right Nickel Salt for Your Application
Nickel sulfate and nickel chloride are both essential nickel salts used in electroplating, with nickel sulfate offering better stability and easier handling due to its lower acidity, making it ideal for general plating applications. Nickel chloride provides higher solubility and enhanced brightness in plating baths, preferred in processes demanding superior metal deposition quality. Selecting the right nickel salt depends on factors such as bath composition, desired plating properties, and process control requirements to optimize performance and efficiency.
Nickel sulfate vs nickel chloride Infographic
 materialdif.com
materialdif.com