Nickel oxide and nickel hydroxide are two important nickel compounds with distinct properties and applications. Nickel oxide (NiO) is a stable, greenish-black powder used mainly in ceramics, batteries, and catalysts due to its high thermal stability and conductivity. Nickel hydroxide (Ni(OH)2) appears as a greenish solid and is widely employed in rechargeable battery electrodes, particularly in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries, because of its excellent electrochemical performance.
Table of Comparison
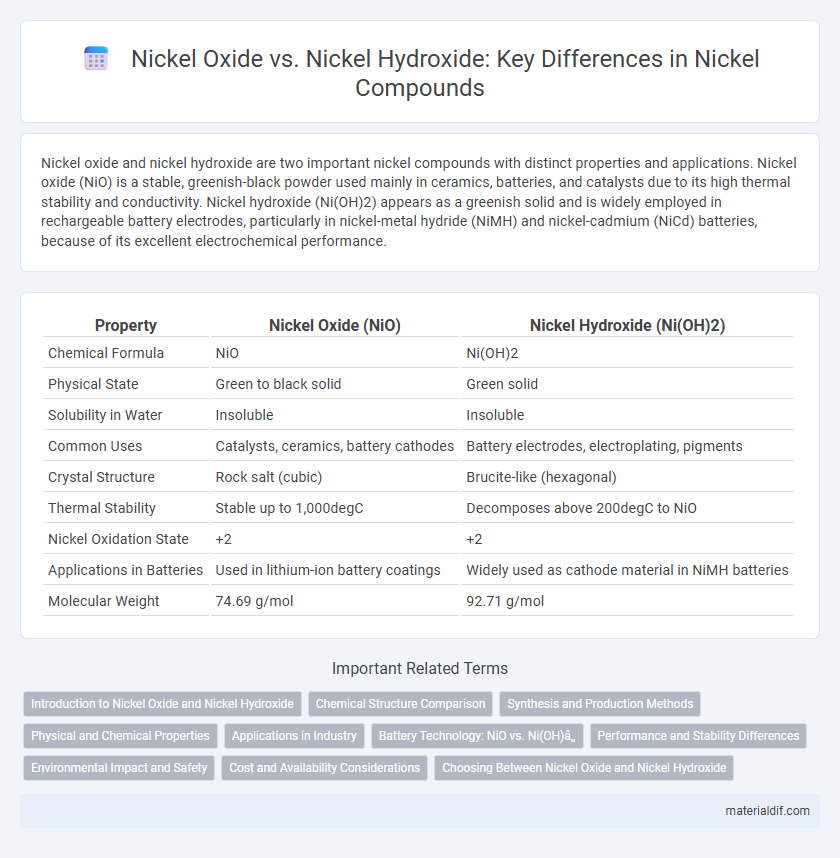
| Property | Nickel Oxide (NiO) | Nickel Hydroxide (Ni(OH)2) |
|---|---|---|
| Chemical Formula | NiO | Ni(OH)2 |
| Physical State | Green to black solid | Green solid |
| Solubility in Water | Insoluble | Insoluble |
| Common Uses | Catalysts, ceramics, battery cathodes | Battery electrodes, electroplating, pigments |
| Crystal Structure | Rock salt (cubic) | Brucite-like (hexagonal) |
| Thermal Stability | Stable up to 1,000degC | Decomposes above 200degC to NiO |
| Nickel Oxidation State | +2 | +2 |
| Applications in Batteries | Used in lithium-ion battery coatings | Widely used as cathode material in NiMH batteries |
| Molecular Weight | 74.69 g/mol | 92.71 g/mol |
Introduction to Nickel Oxide and Nickel Hydroxide
Nickel oxide (NiO) is a stable, green to black inorganic compound commonly used as a catalyst, in ceramics, and battery electrodes due to its excellent chemical stability and electrical conductivity. Nickel hydroxide (Ni(OH)2) is a hydroxide of nickel, typically green and used primarily in rechargeable battery technologies such as nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries because of its high electrochemical activity. Both compounds play crucial roles in energy storage and industrial applications, with nickel oxide offering superior thermal stability and nickel hydroxide providing enhanced electrochemical performance.
Chemical Structure Comparison
Nickel oxide (NiO) consists of nickel ions in a +2 oxidation state bonded to oxygen anions in a rock salt crystal structure, exhibiting a cubic lattice. Nickel hydroxide (Ni(OH)2) features nickel ions coordinated with hydroxide groups (OH-) arranged in a layered hexagonal structure, allowing for intercalation processes. The key chemical structure difference lies in the presence of hydroxyl groups in nickel hydroxide, which significantly influences its electrochemical behavior compared to the simple oxide lattice of nickel oxide.
Synthesis and Production Methods
Nickel oxide is commonly synthesized through thermal decomposition of nickel salts, such as nickel nitrate or nickel carbonate, at high temperatures, resulting in a crystalline oxide form. Nickel hydroxide is typically produced via precipitation methods involving nickel salts and alkaline solutions, leading to layered hydroxide structures ideal for battery applications. Hydrothermal synthesis and controlled pH environments are crucial in tailoring the morphology and purity of both nickel oxide and nickel hydroxide during production.
Physical and Chemical Properties
Nickel oxide (NiO) is a green to black solid with a high melting point of around 1955degC, exhibiting strong ionic bonding and limited solubility in water. Nickel hydroxide (Ni(OH)2) appears as a pale green to brown solid, is less thermally stable, and decomposes upon heating, producing nickel oxide and water. Chemically, nickel oxide behaves as a basic oxide, reacting with acids to form nickel salts, whereas nickel hydroxide acts as a weak base and is commonly used in rechargeable battery electrodes due to its reversible redox properties.
Applications in Industry
Nickel oxide (NiO) is widely used in ceramic glazes, electroceramics, and as a catalyst in chemical reactions due to its thermal stability and conductivity. Nickel hydroxide (Ni(OH)2) primarily serves as the active material in rechargeable battery electrodes, especially in nickel-metal hydride and nickel-cadmium batteries, offering excellent energy storage capabilities. Industries leveraging these compounds benefit from NiO's durability in high-temperature applications and Ni(OH)2's efficiency in energy storage and conversion technologies.
Battery Technology: NiO vs. Ni(OH)₂
Nickel oxide (NiO) and nickel hydroxide (Ni(OH)2) play distinct roles in battery technology, with NiO primarily used as a robust electrode material offering high electrical conductivity and thermal stability. Ni(OH)2 acts as the active material in nickel-based rechargeable batteries, such as nickel-metal hydride (NiMH) and nickel-cadmium (NiCd), due to its excellent electrochemical properties and reversible redox behavior. The choice between NiO and Ni(OH)2 influences battery capacity, cycle life, and energy density, making material optimization crucial for advancing battery performance.
Performance and Stability Differences
Nickel oxide exhibits higher electrical conductivity and thermal stability compared to nickel hydroxide, making it more suitable for high-temperature and high-performance applications such as catalysis and battery electrodes. Nickel hydroxide, while offering superior electrochemical properties and faster charge-discharge cycles, tends to degrade faster under prolonged cycling, reducing long-term stability in energy storage devices. The balance between conductivity and chemical stability drives material selection in electrochemical systems, with nickel oxide favored for durability and nickel hydroxide preferred for rapid redox activity.
Environmental Impact and Safety
Nickel oxide exhibits greater environmental stability and lower solubility compared to nickel hydroxide, reducing its potential for soil and water contamination. Nickel hydroxide poses higher toxicity risks due to its increased solubility and bioavailability, which can lead to greater ecological harm and human exposure through inhalation or ingestion. Proper handling and disposal of nickel hydroxide are crucial to minimize environmental and health hazards associated with its use.
Cost and Availability Considerations
Nickel oxide is generally more cost-effective and widely available than nickel hydroxide due to its simpler production process and greater stability in various industrial applications. Nickel hydroxide, while essential for specific uses like battery cathodes, tends to be more expensive and less readily sourced, impacting its accessibility in large-scale manufacturing. Market fluctuations in raw nickel prices also influence the cost dynamics between these two compounds, with nickel oxide benefiting from broader demand and supply chains.
Choosing Between Nickel Oxide and Nickel Hydroxide
Nickel oxide (NiO) offers high thermal stability and is widely used in catalytic converters and battery electrodes, making it suitable for high-temperature and long-term applications. Nickel hydroxide (Ni(OH)2) features excellent electrochemical properties, primarily used in rechargeable battery cathodes such as nickel-metal hydride (NiMH) batteries, due to its superior capacity for reversible redox reactions. When choosing between nickel oxide and nickel hydroxide, consider the specific application requirements: NiO for durability and heat resistance, Ni(OH)2 for enhanced energy storage and electrochemical performance.
Nickel oxide vs nickel hydroxide Infographic
 materialdif.com
materialdif.com