Electrolytic nickel offers high purity and superior conductivity, making it ideal for electrical and electronic applications. Chemical nickel, also known as electroless nickel, provides uniform coating and excellent corrosion resistance, especially on complex shapes and non-conductive surfaces. Both types serve distinct industrial needs, with electrolytic nickel excelling in conductivity and chemical nickel favored for durability and wear resistance.
Table of Comparison
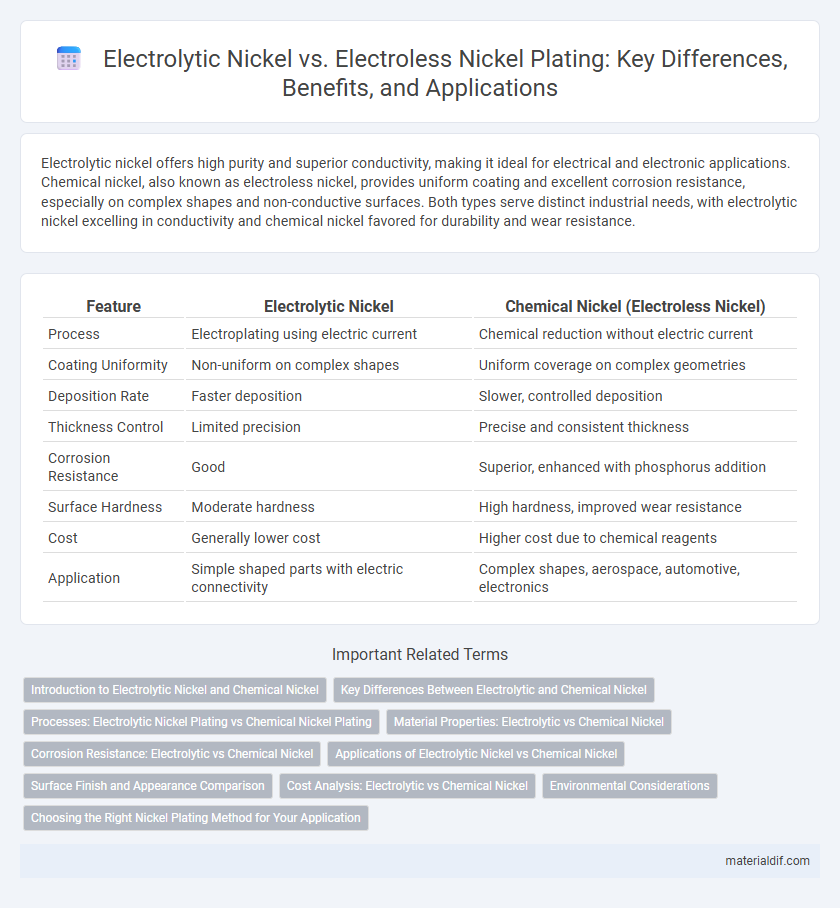
| Feature | Electrolytic Nickel | Chemical Nickel (Electroless Nickel) |
|---|---|---|
| Process | Electroplating using electric current | Chemical reduction without electric current |
| Coating Uniformity | Non-uniform on complex shapes | Uniform coverage on complex geometries |
| Deposition Rate | Faster deposition | Slower, controlled deposition |
| Thickness Control | Limited precision | Precise and consistent thickness |
| Corrosion Resistance | Good | Superior, enhanced with phosphorus addition |
| Surface Hardness | Moderate hardness | High hardness, improved wear resistance |
| Cost | Generally lower cost | Higher cost due to chemical reagents |
| Application | Simple shaped parts with electric connectivity | Complex shapes, aerospace, automotive, electronics |
Introduction to Electrolytic Nickel and Chemical Nickel
Electrolytic nickel is produced through electrorefining, resulting in high-purity nickel with superior corrosion resistance and excellent conductivity, making it ideal for applications in electronics, plating, and batteries. Chemical nickel, also known as electroless nickel, is deposited via a chemical reduction process without electrical current, offering uniform coating thickness and enhanced wear resistance suitable for complex-shaped components. Both forms serve specialized industrial uses, with electrolytic nickel favored for its purity and chemical nickel valued for its uniform deposition and hardness.
Key Differences Between Electrolytic and Chemical Nickel
Electrolytic nickel is produced through an electroplating process that results in high-purity nickel with excellent uniformity, making it ideal for electronics and plating applications. Chemical nickel, also known as electroless nickel, deposits nickel without electricity via a chemical reduction reaction, providing superior corrosion resistance and uniform coating over complex shapes. Key differences include electrolytic nickel's reliance on electric current for deposition versus chemical nickel's autocatalytic process, and their varied uses based on purity, coating uniformity, and corrosion performance.
Processes: Electrolytic Nickel Plating vs Chemical Nickel Plating
Electrolytic nickel plating involves the use of an electric current to reduce nickel ions from a solution onto a metal surface, providing a uniform and controlled deposition ideal for precise thickness requirements. Chemical nickel plating, also known as electroless nickel plating, relies on a chemical reduction process without electric current, resulting in a highly uniform, corrosion-resistant coating even on complex geometries. Both processes enhance surface hardness and wear resistance but differ significantly in equipment complexity, coating uniformity, and application versatility.
Material Properties: Electrolytic vs Chemical Nickel
Electrolytic nickel exhibits high purity levels, typically exceeding 99.9%, resulting in superior electrical conductivity and enhanced corrosion resistance compared to chemical nickel. Chemical nickel, often referred to as electroless nickel, contains phosphorus or boron alloys that provide uniform coating thickness and excellent wear resistance on complex geometries. The distinct microstructure of electrolytic nickel offers better mechanical strength, while chemical nickel's amorphous layer ensures improved hardness and lower friction in industrial applications.
Corrosion Resistance: Electrolytic vs Chemical Nickel
Electrolytic nickel exhibits superior corrosion resistance due to its dense, uniform, and highly pure nickel coating, effectively protecting substrates from oxidation and chemical attack. Chemical nickel, or electroless nickel, provides excellent corrosion resistance through an even, phosphorus-containing alloy layer that enhances durability in harsh environments, especially against wear and corrosion in complex geometries. The choice between electrolytic and chemical nickel depends on application-specific corrosion demands, with chemical nickel favored for uniform coverage and electrolytic nickel preferred for applications requiring high-purity nickel layers.
Applications of Electrolytic Nickel vs Chemical Nickel
Electrolytic nickel is widely used in high-performance applications such as electronics, aerospace, and plating due to its high purity and superior corrosion resistance. Chemical nickel, also known as electroless nickel, is favored for uniform coating on complex geometries and is extensively applied in automotive, oil and gas, and mechanical engineering industries. Differences in deposition methods influence their suitability, with electrolytic nickel offering targeted thickness control and chemical nickel providing consistent coverage and enhanced wear resistance.
Surface Finish and Appearance Comparison
Electrolytic nickel offers a bright, smooth surface finish with high uniformity, making it ideal for decorative coatings and applications requiring excellent appearance consistency. Chemical nickel provides a more uniform deposit on complex shapes, resulting in a matte to semi-bright finish that enhances corrosion resistance without the need for electrical current. Both types deliver distinct aesthetic qualities, with electrolytic nickel preferred for luster and chemical nickel favored for its uniform coverage and durability.
Cost Analysis: Electrolytic vs Chemical Nickel
Electrolytic nickel production incurs higher energy costs due to the electrolysis process, resulting in increased operational expenses compared to chemical nickel plating, which relies on chemical reduction and typically requires less power. Chemical nickel offers cost advantages in terms of lower capital investment for equipment and reduced maintenance, making it more economical for complex shapes and large surface areas. However, electrolytic nickel provides higher purity and superior corrosion resistance, which can justify the higher cost in applications demanding premium material properties.
Environmental Considerations
Electrolytic nickel production involves electrorefining processes that typically consume significant electricity but produce fewer chemical byproducts compared to chemical nickel plating, which relies on chemical reduction methods that may generate hazardous waste. Environmental considerations for electrolytic nickel emphasize energy source sustainability and the management of heavy metal discharge, while chemical nickel's environmental impact centers on controlling toxic chemicals such as hypophosphite and minimizing phosphorus-related effluents. Advanced treatment technologies and regulatory compliance are critical to mitigating pollution risks associated with both electrolytic and chemical nickel production methods.
Choosing the Right Nickel Plating Method for Your Application
Electrolytic nickel plating produces a uniform, hard coating with excellent wear and corrosion resistance, making it ideal for industrial components exposed to harsh environments. Chemical nickel plating offers a more uniform thickness and superior corrosion protection on complex shapes and non-conductive surfaces, suitable for precision electronics and intricate parts. Selecting between electrolytic and chemical nickel depends on factors like substrate material, component geometry, and required coating properties to optimize performance and durability.
Electrolytic nickel vs Chemical nickel Infographic
 materialdif.com
materialdif.com